Table of contents
The US Drug Supply Chain Security Act (DSCSA) sets clear ground rules for what type of information health systems and retail pharmacies need to be able to receive. However, the regulation isn’t nearly as prescriptive in regard to how it should be done—just that the required data is exchanged and available for audit.
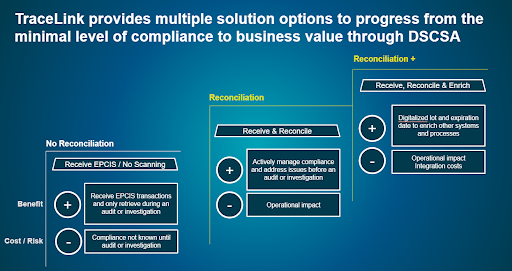
So, what is the best way for dispensers to approach DSCSA 2023 compliance? Should you simply receive EPCIS data, or take the additional steps of reconciling the physical item to the electronic DSCSA compliance information? Or should you go even further, leveraging the digitalized lot and expiration date to enrich other systems and processes?
Our recent webinar, “Balancing Risk and Operational Impact When Complying with DSCSA 2023,” dove into that question. The webinar also covered how your DSCSA 2023 compliance strategy impacts your pharmacy operations and what solutions you can deploy to support that strategy.
Generally speaking, there are three different approaches that most dispensers are taking. The difference between them depends on how your organization wants to balance the risk of noncompliance against the operational impact and integration costs of receiving and reconciling the data. The three approaches are:
- Receiving EPCIS without reconciling it. The benefit of this approach is that it has little operational impact—you simply receive EPCIS transactions and retrieve the data as needed. However,without reconciling the data to and verifying its accuracy, you’ll never know whether you’re compliant until an audit or investigation.
- Receive EPCIS data and reconcile it. With this approach, you’re receiving EPCIS transactions and actively managing your compliance so you can address issues as they occur. However, it has an operational impact, as your employees will need to scan items to reconcile them with the digital data.
- Receive, reconcile, and enrich. This approach enables you to take the digitalized lot and expiration date and use this information to enrich other systems and processes, leveraging the data you’re receiving as part of DSCSA compliance to enrich your pharmacy operations. Some considerations to this approach might include operational impact and costs associated with integrating the data with other internal systems.
Initially, many dispensers opt for the middle approach, receiving and reconciling data as it comes in from wholesale distributors and manufacturers. However, regardless of which approach is ideal for your organization, TraceLink can help.
With TraceLink Product Track, you can reconcile products visually and acknowledge it within the solution. You can also scan and receive products via Smart Inventory Tracker, a mobile solution that synchronously connects with the TraceLink application to verify compliance data. You can even reconcile via an integration with a pharmacy receiving system. It all depends on your standard operating procedure.
If you’re interested in learning more about DSCSA 2023 from a panel of subject matter experts and your industry peers, sign up for the next session in our DSCSA webinar series today. We’ve got a special track dedicated specifically for dispensers.
And if you're curious to see how other dispensers are using TraceLink to empower their approach to receiving and reconciling EPCIS transactions, watch the full webinar. You’ll get two quick case studies looking at a non-profit health system doing $5.4 billion in revenue and a large retail pharmacy chain doing $194 billion.





