Table of contents
The product recall process is time consuming, labor intensive, and unpredictable, with pharmacy teams often struggling to locate recalled product, determine whether the affected product has already been dispensed, and retrieve recalled product still on hand. Delays in this process risk patient safety on top of holding up product reimbursement or replacement.
So, what can the industry do to make managing product recalls easier and more efficient? The underlying cause of recall inefficiency is the manual processes and sub-optimal tools used to manage the end-to-end recall process. Our recent webinar looked at how TraceLink addresses these obstacles with three critical capabilities: Digital Recall Notifications, Targeted Recalls, and Process Orchestration for Empowered Teams (POET) for Product Recalls. Here's how TraceLink helps.
Surface only new alerts to eliminate recall noise
The thought of missing a critical recall notification keeps many pharmacy teams up at night. That’s why the industry fields recall notifications from multiple sources—alert services, the FDA, wholesalers, manufacturers, and more—to ensure they get notified about a recall as soon as possible.
However, this approach isn’t without its drawbacks. Pharmacy teams are often inundated with duplicate recall notifications, receiving eight or more alerts for each recall event. These duplicate alerts still need to be reviewed to verify whether they are net-new recalls or if they contain updates, creating recall “noise” that delays response times.
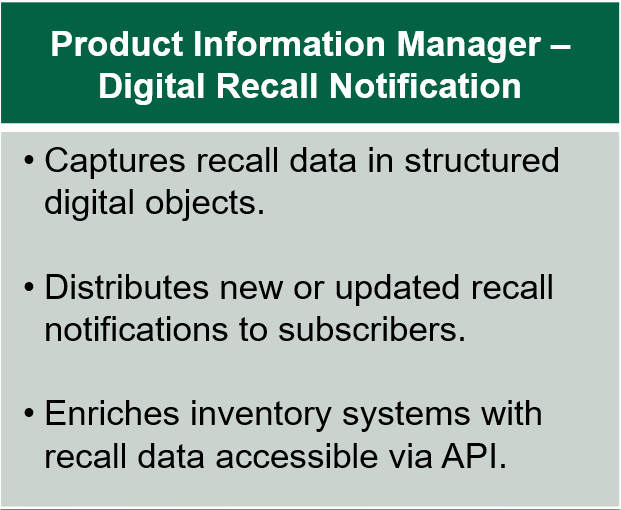
TraceLink PIM-DRN helps health systems and pharmacies by giving them a single source of truth for all recall notifications. The TraceLink Network Services team digitalizes recall incidents from their original source (for example, the FDA website) and transforms them into a structured digital representation. Then, a single notification is sent to each member of the Digital Recalls network when an alert is created or updated, eliminating duplicate alerts and reducing recall noise.
With TraceLink, pharmacy teams no longer have to spend time sorting through dozens of recall notices and manually entering recall information into their enterprise systems. This directly accelerates the initiation of product searches, which is critical given the urgency of product recalls!
Locate where recalled product was received to speed retrieval
Today's pharmacy teams don't have easy access to visibility into what product was received where and in what quantity. They often need to spend critical time in the recalls process compiling spreadsheets using purchase history and the recall notification to determine if their specific site actually received recalled product. Sometimes, they may even skip this step and just start looking for recalled product without knowing if their location received it. The inefficiency of this process will often necessitate multiple shelf walks for each recall event as well to ensure no recalled product was missed.
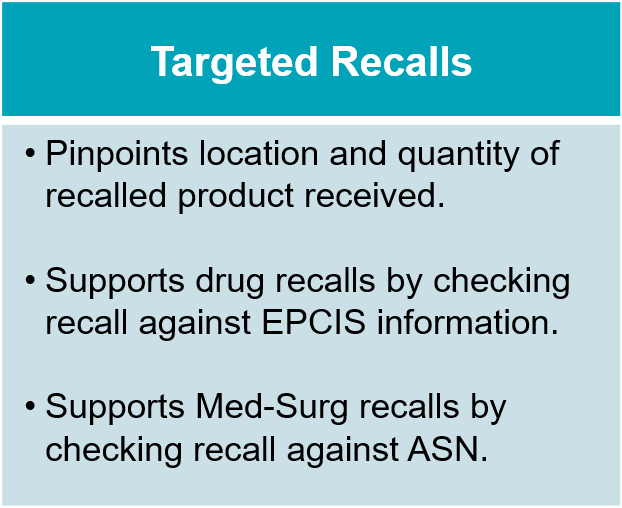
Serialized product data, required for compliance with the U.S. Drug Supply Chain Security Act (DSCSA), transforms this process by enabling specific medicines to be tracked more precisely as they move across the supply chain. With TraceLink, the digitalized recall notification can be crosschecked against serialized receiving data captured by the U.S. DSCSA Compliance solution to quickly surface deliveries containing recalled product. Furthermore, the digitalized recall notice can be accessed by other solutions like medication management systems, granting a more precise location for recalled product.
With this capability (which we call Targeted Recalls), site leads now have the power to instantly determine whether they have received recalled product and, if so, how much. This enables them to eliminate needless product searches, and if they have received recalled product, it gives them a precise quantity of product received to aid shelf walks.
Coordinate recalls across many sites for easier management

Managing and monitoring recall activity across multiple sites gets complicated quickly. In many scenarios, each site will have their own processes for handling recalls and reporting their results back to the recall coordinator. Even in organizations with a more structured approach, communicating with multiple site leads and getting status updates can take hours out of a recall coordinator’s day.
The core issue here is that managing and monitoring recalls often happens over a combination of phone calls, emails, and spreadsheets. Emails and spreadsheets get lost in email inboxes and phone calls go unanswered as pharmacy teams scramble to retrieve recalled product from shelves. As a result, it’s difficult for recall coordinators to get a high-level overview of recall progress and ensure recall procedures are being followed.
TraceLink POET for Digital Recalls makes managing recall activities and monitoring recall progress much easier by consolidating all communication and collaboration into a single platform. This not only makes managing recall workflows more straightforward, it also elevates process visibility and transparency.
Dashboards give recall coordinators a high-level overview of critical information, like how many recalls are in process and how many are past due. Built-in workflows and messaging tools also provide structured processes for site leads to acknowledge recalls and report back their findings. And because the whole process is managed and monitored in one place, all activity is captured digitally to create audit trails and demonstrate process adherence.
Go from chaotic mess to doing more with less with TraceLink
Working with the FDA and many of our customers, TraceLink has created purpose-built capabilities for automating, digitalizing, and streamlining the most labor-intensive and time-consuming steps of the recall process. These capabilities can be used in unison to orchestrate your end-to-end recall process, from receiving the first recall notification to pulling the last recalled product from shelves, helping pharmacy teams reclaim their time for other critical duties.
On our recent webinar, Dan Walles, General Manager of Traceability and Compliance at TraceLink, did a deep dive into how this process looks. Check out his explainer from the webinar to see how TraceLink enables health systems and pharmacies to orchestrate the recall process for faster identification and retrieval of recalled product:

TraceLink also recognizes that pharmacy teams strive to minimize disruption to their processes to ensure patient safety and operational efficiency. That’s why TraceLink Product Information Manager - Digital Recall Notifications and TraceLink Process Orchestration for Empowered Teams (POET) for Product Recalls can be licensed individually. This enables health systems and retail pharmacies to incorporate these capabilities into any existing recall management workflows and solutions they deploy today to further enhance their recall process without disruption.
If you’d like to learn more about our Digital Recall solution, you can watch the recent webinar in its entirety—we cover industry challenges, go over a product demonstration, and give a sneak peek at some upcoming features.
If you want to see how your company can benefit from our Targeted Recalls solution, get in touch with us today. We’d be happy to take a look at your recall process, identify potential process gaps, and uncover potential opportunities.