blue
Product overview
Comply with NMVS serialization and reporting requirements
The TraceLink National Medicines Verification System Country solution provides integration with country-specific NMVS blueprint systems offered by Arvato, SolidSoft, and SecurPharm. It supports NMVS verification and compliance reporting for 3PL, wholesale distribution, hospital, and pharmacy use cases as required under the EU Falsified Medicines Directive (EU FMD).
With NMVS Pharmaceuticals, you can:
Industry challenges
New compliance requirements demand new compliance solutions
Today’s marketing authorization holders and pharmaceutical manufacturers face immense challenges managing regulatory compliance and navigating evolving requirements in the NMVS markets. These challenges include:
Complex product status reporting requirements
After initial reporting of product data to the EMVS, product movement through internal distribution ecosystems and external supply channels may cause product state or status to change, which requires additional reporting.
Complex product status reporting requirements
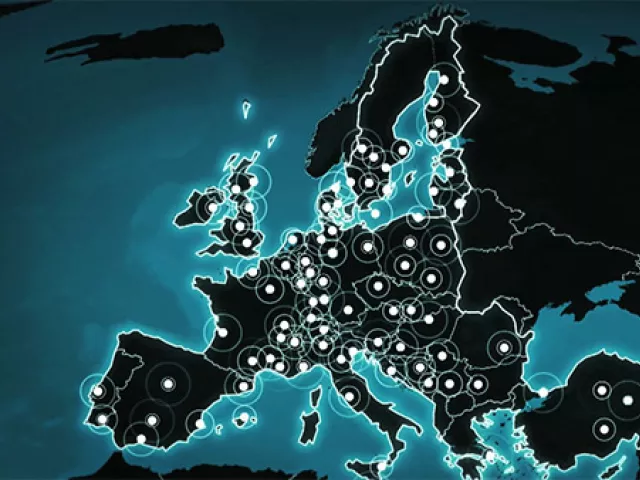
Coordination across multiple national systems
Depending on the specific country in which the product is located or where activity took place, companies must find and report product state changes to the appropriate National Medicines Verification System.
Coordination across multiple national systems
Integration with NMVS to initiate updates
Given the architecture of the EMVS and the diversified nature of country-based requirements as a part of EU FMD, product manufacturers, 3PLs, product distributors, or other parties may be required to connect directly to an NMVS to initiate updates on the product pack information contained in that repository system.
Integration with NMVS to initiate updates
Capabilities
TraceLink NMVS
The NMVS solution provides an advanced compliance data management and reporting system to support diverse product pack, identity, verification, and status management requirements as products move through the supply chain under EU FMD regulations.