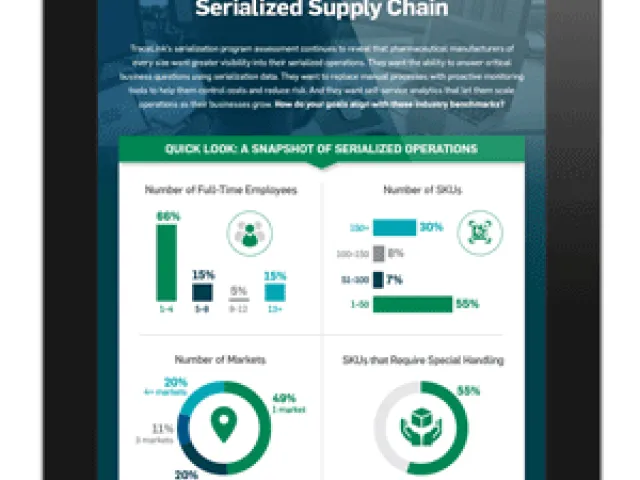
Table of contents
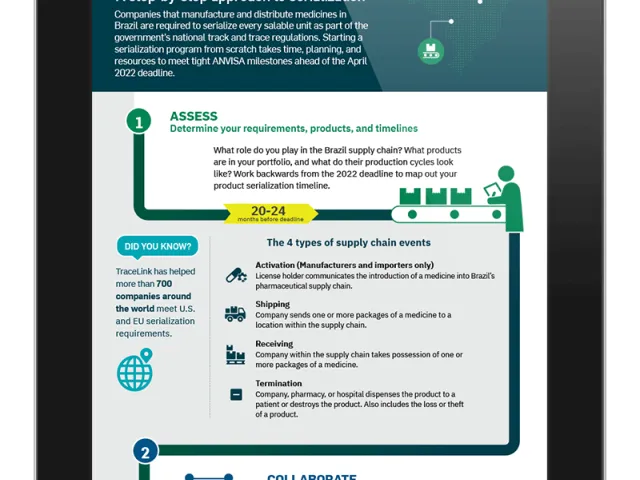
With less than 24 months until the final deadline for meeting Brazil’s industry-wide track and trace requirements, the government’s healthcare regulatory agency, ANVISA, has indicated that companies must have their serialization solutions in place or registered with the agency by the end of this year. Draft normative instruction circulated in May includes several critical targets that affect manufacturers, distributors, and dispensers:
All manufacturers and importers with registered products are expected to serialize and report data for such products to ANVISA by December 1, 2020. It is expected that companies will have some portion of their product lines serialized and activated with ANVISA by the end of the year, and will be able to report to the government system (SNCM) when they begin to ship serialized products.
All manufacturers and importers must register a detailed implementation plan for products that are not yet serialized by December 1, 2020. ANVISA will require that companies provide a complete description of the supply and production ecosystem for every product that must be serialized, including manufacturing plants and packaging facilities; packaging lines; product SKUs; and distribution centers. In addition, plans must include a complete description of the compliance infrastructure for each product, including internal processes; equipment, software purchasing, installation, and validation at the packaging line level; and systems integration with the SNCM. This implementation plan must document delivery of full compliance for any remaining products by February 28, 2022, two months prior to the final compliance deadline.
Wholesalers and most dispensers must be able to process serialized products and begin reporting to ANVISA by December 1, 2020. While some countries, including the United States, are rolling out track and trace requirements to manufacturers, distributors, and dispensers in phases, ANVISA expects that wholesale distributors and retail dispensers will have systems and processes in place for reporting on serialized products as soon as manufacturers start to ship them.
While ANVISA is expected to release the formal publication of its new normative instructions and final technical guidelines in June 2020, the aggressive milestones set in the initial draft of the guidelines are a clear indication that ANVISA expects companies to accelerate their preparations for meeting the Brazil regulations.
The April 2022 deadline has not changed.
The final deadline for meeting all product serialization and government reporting requirements is established by law. ANVISA cannot legally extend the deadline. In fact, according to the draft instructions, ANVISA is requiring that all serialization and reporting solutions must be fully implemented two months prior to the deadline so trading partners can be sure all systems and processes will meet the legal requirements.
Standards organizations like GS1 and Brazil industry associations are already meeting to discuss how companies can implement horizontal integration: the peer-level data exchange necessary to ensure that trading partners have accurate product data for government reporting purposes. TraceLink is very involved in these discussions, and will continue to follow developments in Brazil and provide updates on the latest regulatory and business requirements.
Companies cannot afford to delay their planning and preparation if they are going to meet the December 2020 deadline. Contact TraceLink to learn more about our Brazil track-and-trace solution and how we can help you plan your Brazil serialization and compliance strategy.