Table of contents
With the final implementation phase underway, the deadline for Brazilian track-and-trace regulations to go into effect is May 2022. For companies that are planning their Brazil compliance strategy, this glossary of key regulatory and serialization terms will be a useful reference throughout the journey.
AC-ANVISA—The ANVISA certification authority responsible for issuing digital certificates to SNCM members that are not required to use the standard certificate ICP-BRASIL.
Agent—Person and/or legal entity authorized to communicate with ANVISA on behalf of an SNCM Member.
ANVISA—Agência Nacional de Vigilância Sanitária. The Brazilian Health Regulatory Agency, an autonomous agency reporting to the Brazilian Ministry of Health. ANVISA is responsible for the control of the production, marketing, and use of products and services subject to health regulation, including related environments, processes, ingredients, and technologies, as well as the control in ports, airports, and borders.
ANVISA RDC—Collegiate Board Resolution (Resolução da Diretoria Colegiada) of ANVISA. A numbered and dated resolution issued by ANVISA that provides or updates specific regulatory guidance.
Backend—The computing environment that provides the functionality of SNCM.
Client System—System used by SNCM members for communication with SNCM.
CNPJ—Cadastro Nacional da Pessoa Jurídica. A CNPJ is an identification number issued to Brazilian companies by the Department of Federal Revenue of Brazil (Secretaria da Receita Federal). The CNPJ consists of a 14-digit number formatted as 00.000.000/0001-00. The first eight digits identify the company, the four digits after the slash identify the branch or subsidiary, and the last two are check digits.
Commercial Packing—Usually, the secondary packing (single or multiple) that is considered the “saleable unit” and which must bear a unique IUM. Secondary packaging is normally used to protect the primary packaging, such as tubes and ampules, which is in direct contact with the medicine. In some cases, the primary packing may be the commercial packing.
Consumer—Person who carries out the acquisition of medicines at an authorized dispensation point, such as pharmacies and drugstores (Law No. 5,991/1973, article 6).
Detentor de Registro—The license holder (brand owner) of a pharmaceutical product. Also an entity or organization responsible for packaging of the product or a manufacturer or importer responsible for the registration of the medicinal product for human use regulated by ANVISA (ANVISA RDC No. 157/2017, art. 3, IV).
Dispensation—The act of supplying drugs, medicines, or pharmaceutical ingredients, paid or free of charge (Law No. 5,991/1973, art 4, XV).
Dispenser—Establishment responsible for providing, paid or free of charge, medicinal products to the consumer or patient. Examples: pharmacy, drugstore, hospital, health unit, or health establishment (ANVISA RDC No. 157/2017, art. 3, V).
Distributor— SNCM Member that stores a medicine as an intermediary in any point in the supply chain between the license holder and dispenser (ANVISA RDC No. 157/2017, art. 3, VI).
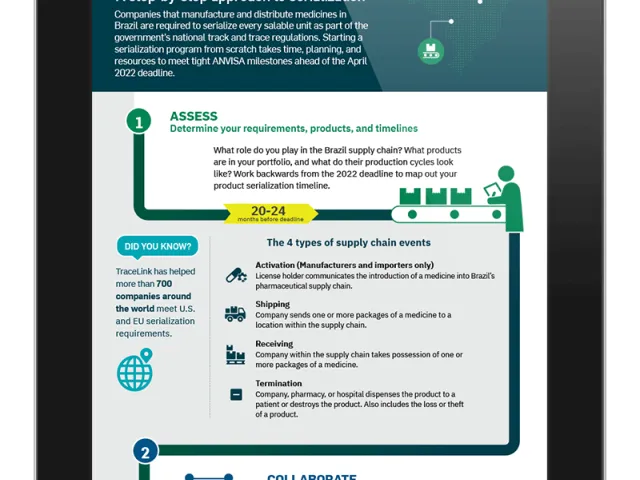
Event Instance—Information related to one Commercial Packing or Shipping Packing that describes an operation/activity that is in the purview of the SNCM. There are 4 event types defined by SNCM:
- Activation: License holders communicate the existence of a medicine that is planned to be introduced in the pharmaceutical supply chain.
- Shipping: Sending one or more packaging of medicinal products to location within the supply chain via transfer, sale, demo, etc.
- Receiving: Receiving one or more packaging of medicinal products to location within the supply chain via transfer, sale, demo, etc.
- Termination: Final step for the product such as dispensation to the patient, destruction of the drug or events like loss or theft.
Event Instance Registration—Recording and storage of an Event Instance within a database belonging to the SNCM Member.
Event Instance Registration Communication—Electronic communication to the centralized SNCM database for an Event Instance registered by the SNCM Member.
ICP-Brasil—Infraestrutura de Chaves Públicas Brasileira. A hierarchical chain of trust that enables the issuance of a digital certificate that identifies and guarantees the identity of a person, company, system or software.
IET— Identificador de Embalagem de Trasporte. Transport packaging identifier (ANVISA RDC No. 157/2017, art. 7).
IUM—Unique Identifier of Medicine (Identificador Único de Medicamentos). The series of standardized alphanumeric, numeric, or special characters that allow the individualized identification of every commercial packing of medication. RDC No. 157 specifies the format and data requirements of the 2D DataMatrix and Human Readable Interpretation of the IUM:

Law 11.903/2009—Federal Law 11.903. The original (2009) law establishing the Brazilian Drug Control System. ANVISA RDC 59/2009 establishes regulations for Federal Law no.11.903. ANVISA RDC 54/2013 amends RDC 59/2009.
Law 13.410/2016—Federal Law 13.410. The current (2016) iteration of the original 2009 law establishing the Brazilian Drug Control System. ANVISA RDC 157/2017 establishes regulations for Federal Law no.13.410.
LCR—List of Revoked Certificates
License Holder—See Detentor de Registro.
Medication Traceability/Track and Trace—Set of mechanisms and procedures that allow one to trace the history, present custody, or last destination of a medication.
Medicine—Pharmaceutical product for curative, palliative, or diagnostic purposes (Law nª 5,991/1973, art. 4, II).
Ministério da Saúde (MS)—The Brazil Ministry of Health.
NTP—Network Time Protocol.
Pharmaceutical supply chain—The flow from the sourcing to consumption of medicinal products, covering the stages of manufacture, import, distribution, transport, storage and dispensation, as well as other types of movement provided for by the sanitary controls (ANVISA RDC No. 157/2017, art. 3, I).
RDC No. 157—ANVISA RDC (Resolução da Diretoria Colegiada) No. 157 defines the Brazilian serialization requirements for the Exploratory Phase. Serialization is required at two levels:
- Unit level: Brazilian IUM applied to each unit of product in a 2D data matrix. Unit-level serial numbers must be unique and random.
- Transport container (case) with one or more medicines: Serialized and aggregated with an identifier linking the container to the unit-level identifiers it contains.
Secretaria da Receita Federal— Revenue Services Secretary. The Department of Federal Revenue in Brazil.
Serial Code—Unique code within the IUM composed of 1 to 20 alphanumeric characters.
Serialization—Generation and application of the DataMatrix and Serial Code on Commercial Packing.
Shipping Packing—Packing container in which multiple Commercial Packages of a medication can be transported.
SNCM—Sistema Nacional de Controle de Medicamentos. The Brazil national drug control system that defines the procedures for drug tracking through technology used to capture, store, and electronically transmit data throughout the pharmaceutical chain.
SNCM Members—Any member of the pharmaceutical supply chain (manufacturers, wholesalers, dispensers) or shipping companies.
Standard Certificate ICP-Brasil—Digital certificate issued by a certification authority accredited by ICP-Brasil.
Timeout—Time interval accepted for return of an electronic communication. Past this interval, communication is considered unsuccessful.
Transport Container—A serialized (aggregated) packaging level that comprises multiple individual serialized units. With aggregation, a serialized identifier can be used to infer the contents of the Transport Container without opening it.
Transporting/Shipping Chain members—Entities responsible for registering Event Instances and sending them to the SNCM database, including: manufacturers, importers, distributers, wholesalers, retailers, hospitals, health clinics, warehouses, merchants, and dispensers.
Unitization of Doses of Medicine—Procedure carried out under the guidance of a pharmacist for the preparation of unit doses to meet the therapeutic needs of patients.
Unseal—Opening a multiple or hospital packing by an SNCM member.
UTC—Coordinated Universal Time.
Web Services—Web technology for providing communication services for SNCM, which allows the exchange of information between the client system and ANVISA.
Global serialization terms
This list includes terms commonly used in the U.S., EU, and other countries and regions where serialized product identifiers are required.
2D DataMatrix—The GS1 DataMatrix is a two-dimensional, or 2D, barcode which may be printed as a square or rectangular symbol that uses patterns of squares, hexagons, dots, and other shapes to encode data. Because of their structure, 2D barcodes can hold more data than 1D “linear” barcodes, while still appearing physically smaller.

3PL—Third-Party Logistics. A contracted company that provides distribution services of finished goods on behalf of another company.
3PP—Third Party Printer. An organization that’s contracted to print serial numbers onto packaging containers.
ADR—Authorized Distributor of Record. A wholesale distributor that a manufacturer designates or authorizes to distribute its products.
Aggregation—The process of recording the serial number of a container along with the serial numbers of its contents; often referred to as a parent/child relationship, or a serialized container to content relationship. See SSCC.
Alphanumeric—Character set made up of digits and letters of the alphabet.
AS2—Applicability Statement 2. Protocol used to securely transmit data over the Internet. Preferred method for exchanging Electronic Data Interchange (EDI) transactions.
ASN—Advance Ship Notice, the common name for the EDI 856 transaction. A notification of pending deliveries, usually in an electronic format.
Asynchronous Transactions—Transactions that do not have to be completed before another transaction can be processed.
Authenticate—The practice of checking a unique identifier against a set of captured serialized data to determine its authenticity.
Auto ID—Methods for automatically capturing data encoded on items and containers, and entering that data directly into computer systems (i.e., without human involvement).
B2B—Business-to-Business. Interactions that support the transfer of standardized interchange files up to an enterprise’s EDI system. B2B interactions are not integrated with manufacturing, warehouse, or other backend business systems.
Batch—A group of products, usually associated by a manufacturing or packaging operation. Also referred to as a lot.
BoL—Bill of Lading. A document issued by a carrier which details a shipment of merchandise and gives title of that shipment to a specified party.
Bundle—A group of items held together, usually by shrink wrap. See also Inner Pack.
Bright Stock—An approach to labeling and packaging operations in which products are produced in large batches, stored in unlabeled containers, and then labeled just prior to shipment.
CAPA—Corrective And Preventive Action. Improvements to an organization’s processes taken to eliminate quality failures.
Case—A container of product cartons which may or may not be bundled. A case-level container that comprises multiple individual serialized units can be also be serialized to enable aggregation. See also Transport Container.
CBV—Core Business Vocabulary. Vocabulary elements agreed upon by trading partners who will exchange data. Example: For serialization, the GS1 standards organization publishes a CBV for EPCIS event data exchange (More at www.gs1.org/epcis).
Check Digit—Redundancy check used for error detection of identification numbers. Used in NDCs, DEA numbers, GTIN-14 identifiers, and SSCCs.
CMO—Contract Manufacturing Organization. A company providing manufacturing, and sometimes packaging, services for one or more companies based on contracts or service agreements. Also referred to as a Contract Packaging Organization (CPO) or Third Party Manufacturer (TPM).
Commission—Process of associating a unique identifier to a particular object (product, shipment, asset, or container).
Counterfeit—An imitation usually created with the intent of fraudulently passing it off as genuine, often to take advantage of the established worth of the imitated product. The word counterfeit frequently describes forgeries of currency and documents, and the imitations of clothing, software, pharmaceuticals, jeans, watches, electronics, and company logos and brands. In the case of goods, it results in patent infringement or trademark infringement.
CPO—Contract Packaging Organization. A third-party organization that manufacturers will subcontract to package their product.
CSV—CSV Comma Separated Values. A common data exchange format stored in a tabular format. CSV files can be opened in spreadsheet programs.
Data Carrier—A GS1 term for the different kinds of media, such as barcodes, that can hold GS1 identification keys and application identifiers.
DC—Distribution Center. A warehouse stocked with goods to be redistributed to retailers, wholesalers, or customers.
Decommission—The process of removing a unique identifier from a product or container so it is no longer tracked. Unlike the business process known as destroying, the item may still physically exist after decommissioning even though it no longer carries serialized identification.
Destroy—In instances where a product or container no longer exists, the process of removing a unique identifier from that item so it is no longer tracked.
Disaggregation—Removing products or containers from their associated parent container. The serial numbers of the contained items are no longer associated as children of the parent container.
Dispenser—A retail pharmacy, hospital pharmacy, group of chain pharmacies, or any other person authorized by law to dispense or administer prescription drugs. Under DSCSA, an entity is not considered a dispenser if it acts as a wholesale distributor or dispenses products only used for animals.
Disposition—The state of a serial number, such as commissioned or decommissioned.
Double Byte Character Set—Character encoding that contains a large number of unique characters or symbols used to express languages such as Japanese, Korean, and Chinese.
Downstream—The direction in which product flows in a supply chain. Generally speaking, pharmaceutical products flow, and transactions occur, through the supply chain from manufacturers, to repackagers, to wholesale distributors, to dispensers.
ECC—Error Correction Coding. A code applied to transferred data for error control. Provides redundancy and allows the receiver to recover the original data.
EDI—Electronic Data Interchange.The electronic transfer of data between computer systems in a standardized message format.
Encoding—A state for serial numbers. If an organization pre-prints labels for serial numbers after they are provisioned, the serial numbers enter the encoding state. Once the labels are affixed to the products, the serial numbers enter the commissioned state.
EPC—Electronic Product Code. A unique number that identifies a specific item in the supply chain. Also known as a serial number.
EPCglobal—The organization developing standards for the Electronic Product Code (EPC), and for RFID systems to store and manage EPCs. EPC Global is sponsored by GS1.
EPCIS—Electronic Product Code Information Services. A GS1 EPCglobal standard designed to enable EPC-related data-sharing within and across enterprises. This data-sharing is aimed at enabling participants in the EPCglobal Network to obtain a common view of the disposition of EPC-bearing objects within a business context. (More at www.gs1.org/epcis).
ERP—Enterprise Resource Planning system. Business process management software used to manage and automate back-office operations.
Event Repository—A computer system designed to store serial number information, and events relating to serialized products.
Exclusive Distributor—A wholesale distributor that purchases directly from a manufacturer and is the sole distributor of that manufacturer’s product.
Expiry—Date of expiration for an item, or the last day the item should be used.
Extension Digit—A one-digit segment used to extend the serial reference segment of an SSCC identifier.
External Product Identifier—A standards-based product code, such as a Global Trade Item Number (GTIN), or a market-specific product code used to identify the product in the external supply chain. This is specifically not a manufacturer SKU, which is not regulated or standardized.
Filter Value—A one-digit value that companies specify in serial number formats. Typically, companies specify filter values as units of measure in order to convert to different formats.
Functional Specification (FS)—In systems engineering and software development, a functional specification (also, functional spec, specs, functional specifications document [FSD]) is the documentation that describes the requested behavior of an engineering system. The documentation typically describes what is needed by the system user, as well as requested properties of inputs and outputs (e.g., of the software system).
GCP—Global Company Prefix. A globally unique code that is used to represent a location in identifiers. See also GS1 Company Prefix.
GLN—Global Location Number. A unique 13-digit number containing a GS1 company prefix, a location reference, and a check digit, used to uniquely identify a physical location or legal entity in the supply chain. The GLN makes possible the unique and unambiguous identification of those locations and entities.
Global Identifier—A unique reference number used to identify a legal entity such as a company or location, to support the secure exchange of business information on the Internet.
Grandfathering—A provision in which some pre-existing situations are not subject to a new rule or regulation.
GS1—A leading global organization dedicated to the design and implementation of global standards and solutions to improve the efficiency and visibility of supply and demand chains globally and across sectors. The GS1 system of standards is the most widely used supply chain standards system in the world. (More at www.gs1.com).
GS1-128—A linear barcode, formerly referred to as a Code-128 barcode. Usage is granted to organization members of GS1. See Linear Barcode.
GS1 Company Prefix—A globally unique identifier for a company, assigned and administered by GS1 Global. The GS1 Company Prefix is 4 to 12 digits, and is a component of GLN, GTIN, and SSCC identifiers.
GS1 Datamatrix—A two-dimensional matrix barcode consisting of black and white “cells” or modules arranged in either a square or rectangular pattern. The information to be encoded can be text or raw data. Usage is granted to organization members of GS1.
GTIN—Global Trade Item Number. An identifier for trade items, developed by GS1. Such identifiers are used to look up product information in a database, often by inputting the number via a barcode scanner pointed at an actual product. The uniqueness and universality of the identifier is useful in establishing which product in one database corresponds to which product in another database, especially across organizational boundaries. Usage is granted to organization members of GS1.
GxP—Good Practice. A general term, usually referring to quality and regulations. For example, GMP is “Good Manufacturing Practice” and GCP is “Good Clinical Practice.”
Header—Headers comprise the prefixes used in Human Readable Interpretation of variable data. Common headers include GS1 Application Identifiers (AIs) or GS1 recommended field labels.
HRI—Human Readable Interpretation. Characters, such as letters and numbers, which can be read by people and are encoded in data carriers. HRI is a one-to-one illustration of the encoded data.
Inference—The technique of assuming the serial numbers within a sealed container based on previous observation, and not by directly reading each serial number. Inference is accomplished using data systems or documents and is controlled through validated procedures.
Inner Pack—A group of items held together, usually by shrink wrap. See Bundle.
Inspection—The process of reviewing an item, either manually or using automated systems.
Internal Material Number—A number assigned to a product for internal use and not for identifying the product externally.
Interoperability—The ability of technology systems and software to communicate, exchange data and/or information, and make use of the information that’s been exchanged.
IQ—Installation Qualification. IQ demonstrates that a process or equipment meets all specifications, is installed correctly, and that all the required components and documentation needed for continued operation are installed and in place.
Item—The product secondary package level, typically a carton. Also referred to as the smallest saleable unit.
L1 to L5—The 5 levels of serialization and information management as defined by the ANSI/ISA-95 automation standard:
- L5 – Network-level serialization system
- L4 – Enterprise serialization system
- L3 – Site-level serialization
- L2 – Packaging line software
- L1 – Device
LDAP—Lightweight Directory Access Protocol. An industry standard protocol for accessing and maintaining distributed directory information services.
Linear Barcode—Also known as a 1-dimensional or 1D barcode. Linear barcodes use a series of variable-width lines and spaces to encode data. Linear barcodes hold just a few dozen characters, and generally get physically longer as more data is added.

LMS—Line Management System. A system that manages a production line and interfaces with a company’s Enterprise Resource Planning (ERP) system.
Logistic Labeling—Comprises the data and process of printing labels for use on all containers above the secondary packaging level. For example, shipper and pallet labels.
MAH—Marketing Authorization Holder. The license holder (brand owner) of a pharmaceutical product. An entity or organization responsible for packaging of the product.
Master Data—Data representing a company’s details, global identifiers, products, and trading partners. Particular types of data are required for serialization and global compliance reporting.
MES—Manufacturing Execution System. A control system for managing and monitoring work-in-process on a factory floor.
National System—An information system set up and governed by national stakeholders to ensure a medicine’s authenticity by verifying its safety features and to prevent falsified products from entering the supply chain.
NDC—National Drug Code. A unique 10-digit product identifier for human drugs in the U.S. that represents the labeler or vendor, the product, and the package size. In Brazil, the ANVISA code plus the GTIN serve as the national drug code.
OEE—Overall Equipment Effectiveness. Evaluation of the effectiveness of a manufacturing operation.
OQ—Operational Qualification. OQ demonstrates that all facets of a process or equipment are operating correctly.
Pack Marking—Comprises the data and process for printing on primary and secondary product packaging.
Packaging and Labeling—Generally, related to the physical material, artwork, and printing that’s used with all levels of product and logistics containers.
Pallet—A flat transport structure (sometimes called a skid) that supports goods in a stable fashion while being lifted by a forklift, pallet jack, front loader, or other jacking device. A pallet is the structural foundation of a unit load, which allows handling and storage efficiencies. Goods or shipping containers are often placed on a pallet secured with strapping, stretch wrap, or shrink wrap, and then shipped.
Parallel Importer—An organization that buys a product on the open market with the intention to repackage or relabel, and then distributes it outside the network that’s set up by the manufacturer or that manufacturer’s authorized distributor.
Picking—The process of collecting articles in a warehouse to fulfill a customer order.
PQ—Performance Qualification. The documented evidence that a system, equipment, or process is capable of consistently producing a safe product of high quality. Performance Qualification protocol describes the procedures that verify the specific capabilities of a process equipment/system through the use of simulation material and/or actual product.
Primary Package—Primary containment system in which the product is sterilized (excluding shelf cartons and shipping containers) to protect the contents to the intended level over a specific period of time. The primary containment system is not usually the Smallest Saleable Unit and does not require a unique identifier. See Smallest Saleable Unit.
Product Code—A unique identifier assigned to each finished manufactured product that is ready to be marketed or sold.
Recall—The removal of a drug product from the market.
RFID—Radio-Frequency Identification. The use of an object, typically referred to as an RFID tag, applied to or incorporated into a product, animal, or person, for the purpose of identification and tracking using radio waves.
Safety Features—Elements, such as anti-tampering devices and barcodes carrying product and pack data, that are incorporated into a medicine product’s packaging and identification to facilitate verification.
SAN—Storage Area Network. A network that provides access to consolidated, block-level data storage.
SCAC—Standard Carrier Alpha Code. Code used to identify transportation companies.
SDB—Serialization database. A level 4 serialization system.
Secondary Packaging Level—Usually, the smallest saleable unit that must bear a unique serialized identifier as compared to a Primary Package such as a v, a blister pack, or bottle.
Segment—Part of a market or industry. The pharmaceutical supply chain includes segments such as manufacturer, wholesaler, dispenser, contract manufacturing organization (CMO), third-party logistics (3PL) companies, and repackagers (also known as third-party packagers or 3PPs).
Serial Number—Typically a portion or component of a Unique Identifier (UID) which provides uniqueness. Also known as a serial reference.
sFTP—Secure File Transfer Protocol. A network protocol that provides file access, transfer, and management over a secure channel.
SGLN—Serialized Global Location Number. A unique identifier to a physical location, such as a specific building or bin within a warehouse. The GLN is a GS1 format; the SGLN is an EPC format and is represented in Uniform Resource Identifier format, for example: urn:epc:id:sgln:0030001.12345.400.
sGTIN—Serialized Global Trading Item Number. The combination of a Global Trade Identification Number and a serial number which uniquely identify an item.
Site Server—A computer system located in a specific locale responsible for a location-specific function. In traceability systems, site servers usually refer to local servers which allocate serial numbers to packaging control systems and/or manage serial number information before it is transmitted to an enterprise traceability event repository.
SKU—Stock Keeping Unit. Specifies a distinct type of item for sale. SKUs are not regulated or standardized and thus are not used for serialization.
Smallest Saleable Unit—The smallest quantity of a product intended for individual sale that must bear a unique, serialized product identifier. Typically described as the secondary packaging level.
SNI—Standardized Numerical Identifier. A standard identifier affixed to a prescription drug package.
SOAP—Simple Object Access Protocol. A messaging protocol for exchanging structured (XML) information in the implementation of web services.
System of Record—An information storage system that is the authoritative data source for a given data element or piece of information.
SSCC—Serial Shipping Container Code. A GS1 standard used in logistic encoding and communications. The SSCC ensures that logistic units are identified with a number that is unique worldwide. See Aggregation.
SSO—Single Sign On. A session and user authentication service that allows software system users to log in with a single username and password, to access connected systems without using different usernames and passwords.
Synchronous Processing—Type of processing that provides an immediate response to a query. SOAP and REST web services provide synchronous processing.
TPO—Third-Party Organization. Term used to refer to any TPM, 3PL, CMO, or other externally contracted organization.
Track and Trace—The process of tracking drugs through the supply chain using serialization data. Track and trace systems begin with serialization but generally include additional components such as product tracing or tracking, verification, and/or reporting.
UID—Unique Identifier. A string of numbers and characters that is unique within a given system. Examples include GS1 GTIN and GS1 SSCC identifiers.
UPC—Uniform Product Code. A form of GTIN data carrier or barcode.
Upstream—The opposite direction that product flows in a supply chain; moving back up the supply chain. Generally speaking, the pharmaceutical product flows, and transactions occur, through the supply chain from manufacturers, to repackagers, to wholesaler distributors, to dispensers.
User—An entity, individual, or organization responsible for making use of product, process, or systems.
Validation—Documented procedure for obtaining, recording, and interpreting the results required to establish that a process will consistently yield product complying with predetermined specifications.
VAN—Value Added Network. A hosted service offering that acts as an intermediary between business partners sharing standards-based or proprietary data, via shared business processes.
Virtual (Manufacturer)—A company that outsources services to a manufacturer/CMO.
Wholesale Distributor—A company that distributes drugs to an entity other than a consumer or patient.
WMS—Warehouse Management System. A software application that supports the day-to-day operations within a warehouse. A WMS enables centralized management of tasks such as tracking inventory levels and stock locations.
WSDL—Web Service Definition Language. An XML-based interface used to describe the functionality of a web service.
XSD—XML Schema Definition. Describes the structure of an XML document