Table of contents
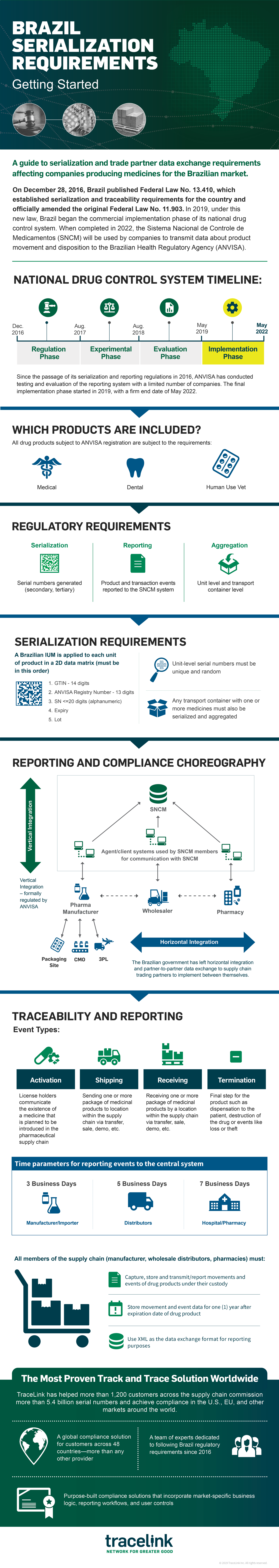
In 2019 Brazil began the commercial implementation phase of its national drug control system. When completed in 2022, the Sistema Nacional de Controle de Medicamentos (SNCM) will be used by companies to transmit data about product movement and disposition to the Brazilian Health Regulatory Agency (ANVISA). This visual guide to serialization and data exchange requirements gives companies producing medicines for the Brazilian market a quick understanding of the basics of Brazil regulations. Learn more about:
- Timelines
- Which products are included
- Regulatory and serialization requirements
- Government reporting