Table of contents
The deadline for complying with the final phase of the U.S. Drug Supply Chain Security Act (DSCSA) is November 27, 2023. With less than 10 months to go, that means it's time for your company to get ready.
Some organizations in the pharmaceutical and healthcare supply chain may not be aware of what the DSCSA compliance requirements are or how these regulatory requirements apply to their specific business. And many more haven't started preparing to meet these compliance requirements yet. This article will delve into DSCSA 2023, its goals, and how you can get started with TraceLink.
What is DSCSA?
DSCSA is a law designed to protect patients by securing the U.S. pharmaceutical supply chain against counterfeit or altered medicines. It does this by requiring interoperable, electronic tracing of products as they make their way from manufacturer to patient.
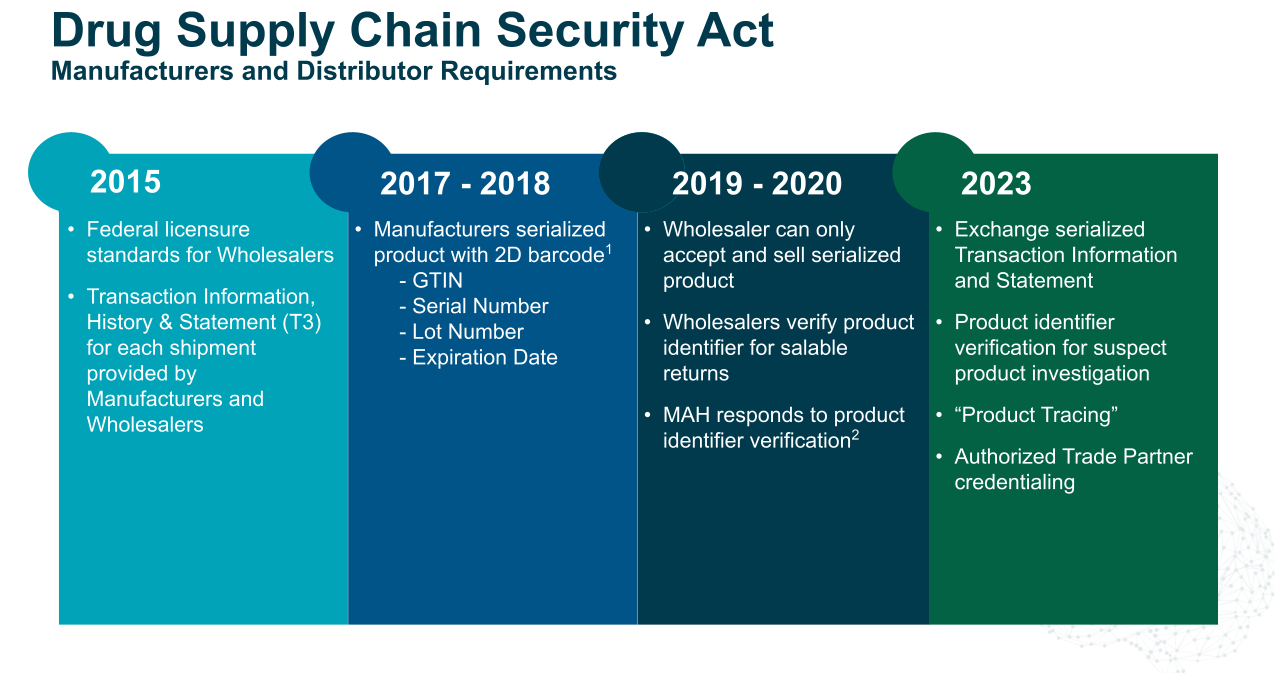
While the compliance requirements are clear, how they are implemented on a technical level is not. That's why the Partnership for DSCSA Governance (PDG), a collaborative public-private partnership dedicated to developing, advancing, and sustaining a model for interoperable product tracing, has created a framework for the implementation of interoperable systems and processes.
The PDG "Interoperability Blueprint" breaks DSCSA 2023 interoperability requirements into three specific but highly interrelated statutory components:
Interoperable exchange: Trading partners must be able to exchange required Transaction Information (TI) and Transaction Statement (TS) data in a secure, electronic, interoperable manner. This TI must include critical product identifiers at the package or item level, including serial number and Global Location Number (GLN).
Interoperable verification: Trading partners must be able to verify the product identifier on a package or sealed homogeneous case in a secure, electronic, interoperable manner.
Interoperable tracing: Trading partners must maintain secure, electronic, interoperable systems and processes to provide TI and TS in response to trading partner or FDA requests for it. They must also have the capability to promptly gather the necessary information to produce the TI for each transaction going back to the manufacturer.
How TraceLink Helps Meet DSCSA 2023 Compliance Requirements
TraceLink developed its industry-leading track-and-trace solutions on a network platform because it was the most efficient design to support the full intent of the DSCSA law: to protect the U.S. drug supply chain from end to end. This forward-thinking approach has not only produced the most comprehensive solution, but it is also the fastest to deploy with the lowest total cost of ownership.
TraceLink helps minimize DSCSA 2023 compliance costs in four critical areas by providing:
- A proven network that supports 100% interoperable data exchange: A network-based model is the most efficient way to exchange data with hundreds or thousands of trading partners. TraceLink "Integrate Once, Interoperate with Everyone™" requires only a single integration to the TraceLink network to send EPCIS data and support product verification. The TraceLink network already has more than 290,000 members, which may include many or all of your partners. More than 900,000 EPCIS transactions have already been exchanged between network members. This is the fastest path to 100% interoperable data exchange.
- A scalable cloud architecture to meet higher transaction volume: Interoperable exchange of TI transactions in EPCIS format is a major step change from the current lot tracing mandate. The TraceLink network and DSCSA compliance solutions are built on a highly scalable cloud platform to process data-intensive DSCSA transactions at operational scale at no additional cost.
- A simplified compliance approach: TraceLink continuously monitors evolving DSCSA and other regional compliance requirements, standards, and partner requirements to update our solutions as required. New compliance requirements are applied across the network so that all trading partners are upgraded at once and out-of-sync transactions are eliminated. This greatly reduces the costs associated with the resources required to monitor compliance requirements, industry approaches to meet these requirements, update integrations, and solutions.
- A resilient architecture to ensure supply: TraceLink solutions run on a platform with an active-active architecture, providing zero recovery time and no data loss to ensure shipments are not delayed by late, missing, or corrupt EPCIS transactions. Drug product shipments cannot be received into inventory and dispensed to patients unless the physical serialized product matches the EPCIS sent by the supplier.
If you want to learn more about DSCSA 2023 and how TraceLink can help you meet mandated compliance goals, watch "4 Must-Have Capabilities for DSCSA 2023 Compliance," a recent webinar we conducted on the subject. Arm yourself with the expert insights you need to get ready now and turn DSCSA compliance into a competitive advantage
Start Preparing for DSCSA 2023 Today
TraceLink has a global services team that can quickly execute a DSCSA readiness assessment and recommend the necessary steps to achieve compliance by November 27, 2023. Contact your TraceLink Account Executive or Services Project Manager to get this project started now or email us at DSCSA [at] tracelink.com.
DSCSA [at] tracelink.com ()
Build Your Track-and-Trace Compliance Foundation at FutureLink
FutureLink is the only supply chain event designed for pharma industry leaders. DSCSA 2023 and other global track-and-trace mandates feature prominently at FutureLink 2023, so join us May 22-24 and discover how to lay a flexible compliance foundation that empowers your future global growth.





