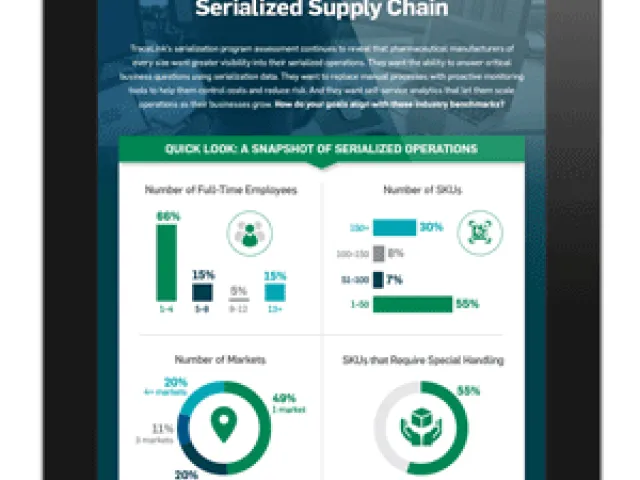
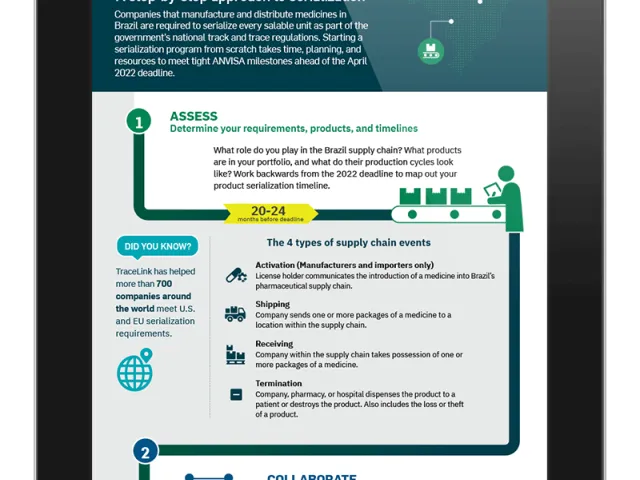
Table of contents
One of the leading pharmaceutical companies in India operates in a complex manufacturing and distribution environment—in addition to managing manufacturing and shipping processes together in one facility, they also ship products globally, including to the United States (US) and the European Union (EU). If not properly managed, everyday occurrences like shipping changes or damaged product can trigger an avalanche of headaches, risking precious time, resources, and worse, introduce potential errors to data required for compliance.
More than three years ago, the manufacturer selected TraceLink as their Level 4 serialization and compliance partner, and approximately two years ago, they implemented TraceLink's flexible Edge solution, which helps them...