Table of contents
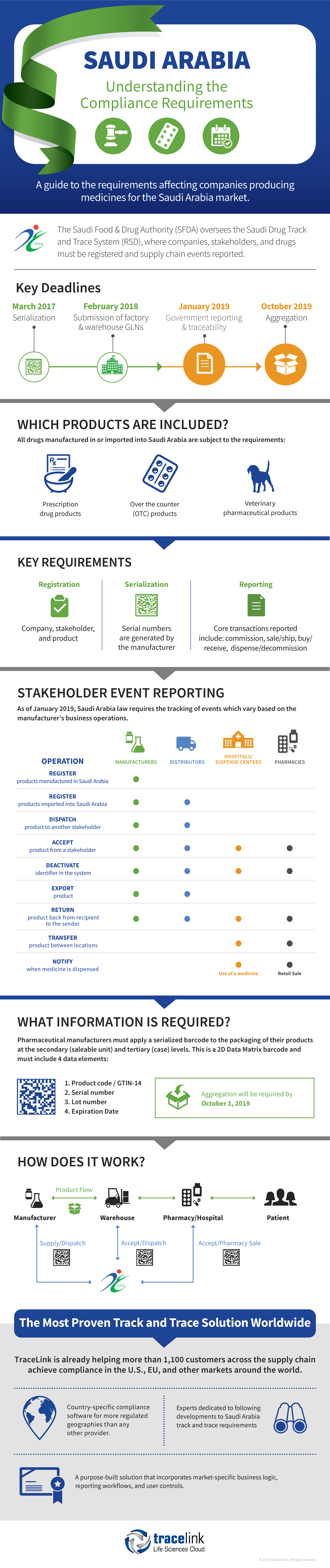
Regulatory update: The Saudi government has notified registered users of the goverment's drug track and trace system (RSD) that the deadline for aggregation of medicines is now August 20, 2020.
On January 7, 2019, the Saudi Food and Drug Authority (SFDA) began enforcement of reporting requirements that affect all companies producing medicines for the Saudi Arabia market—and more deadlines will be coming later this year. This infographic provides a high-level overview of key deadlines, roles and responsibilities, packaging elements, and more, to help you understand the requirements and how they will impact your business.
As the trusted compliance partner of more than 1,000 companies around the world, TraceLink closely monitors regulatory laws and guidance to deliver purpose-built solutions for meeting today’s—and tomorrow’s—track and trace regulations.