Targeted Recalls
Thank you for contacting us; we’ll be in touch shortly.

Point-to-Point Predicament: Why a Network Approach Matters for Reducing DSCSA Costs & Accelerating Digitalization
DSCSA 2023 isn’t just about sending EPCIS transactions—its item-level traceability requirement will require a tremendous shift from all members of…

Pharmacies and Recalls: Understanding the Impact—and Changes to Come
Read about how drug-related recalls affect pharmacies' operational efficiency, how to be proactive on recalls, and how serialization can help.

Fixing Recalls: FDA Pilot Tackles a Legacy Challenge—7 Broken Practices and 5 Guiding Principles for Change
Seven broken product recall practices across the end-to-end pharma supply chain—and five guiding principles for change.

Faster Recalls, Better Visibility: How Serialized Inventory Can Transform Your Health System
Faster recalls and better visibility lead to improved patient care. Learn how the right approach to DSCSA compliance can do just that.

FDA Pilot Report: Digital Recalls Network and DSCSA 2023 Traceability with Blockchain
See the results of TraceLink’s FDA pilot project that focused on two workstreams; digital recalls and blockchain with participants from 22 companies…
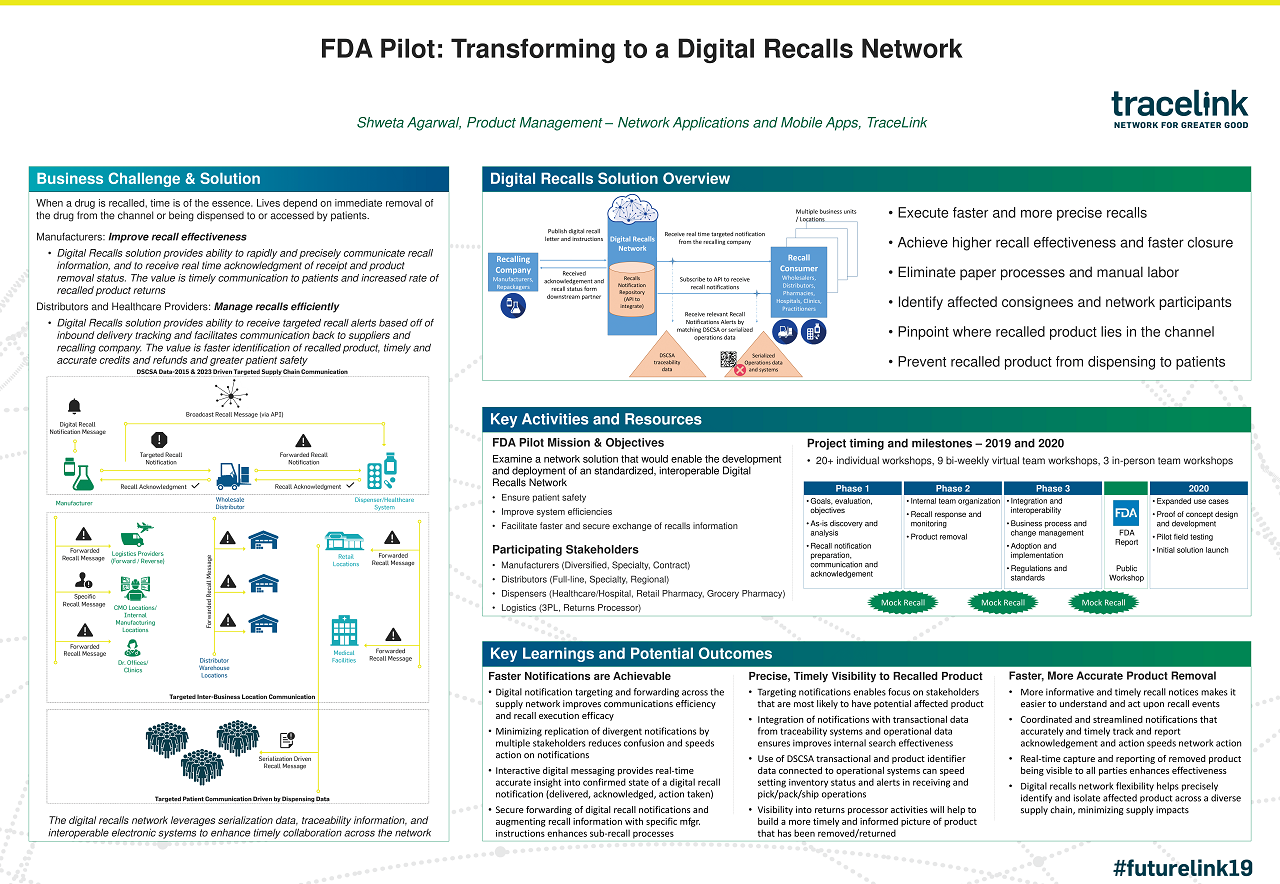
Case Study: TraceLink | FDA Pilot - Transforming to a Digital Recalls Network
Find out how TraceLink helps pharmaceutical manufacturers and dispensers manage recalls more quickly and efficiently than ever.

Case Study: PharmaLink | Closing the Gap on Cradle-to-Grave Traceability via Reverse Distribution and EPCIS
Learn how pharma returns specialist PharmaLink increased pharma supply chain security by combining decommissioning and secure product disposal.


