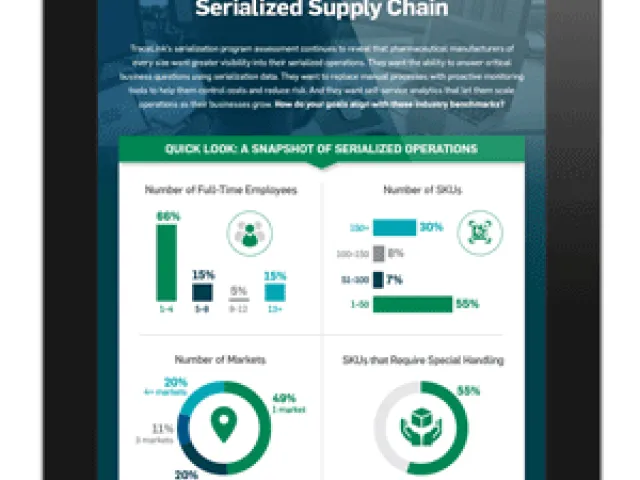
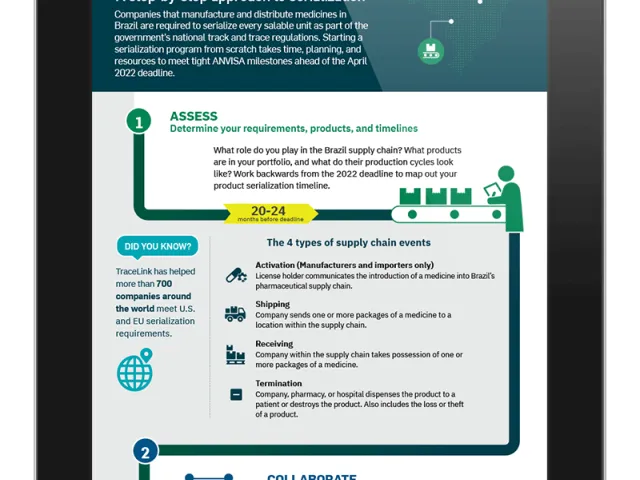
Table of contents
Regulatory update: The Saudi government has notified registered users of the goverment's drug track and trace system (RSD) that the deadline for aggregation of medicines is now August 20, 2020.
TraceLink Vice President of Industry Marketing Brian Daleiden discusses the advantages of having a global approach to compliance—and how Saudi Arabia can fit into your strategy.

As the leading provider of track and trace solutions for the pharmaceutical industry, TraceLink’s proven compliance platform and integrated digital supply network provide a purpose-built foundation for meeting Saudi regulations and give companies a significant advantage over implementing a customized solution.