Table of contents
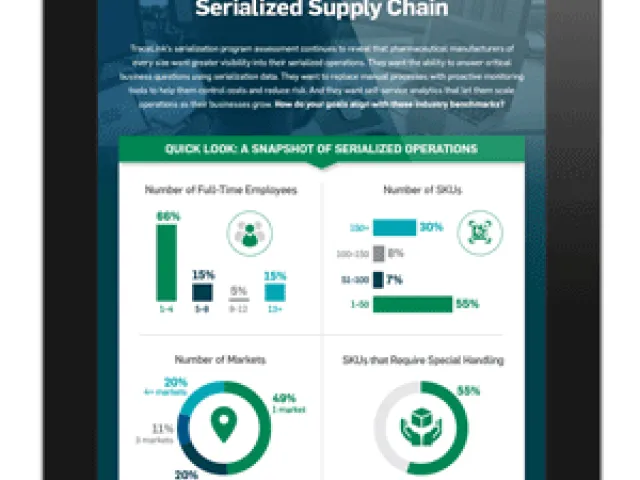
How have companies that sell product into the U.S. adapted to DSCSA lot-level requirements, and how prepared are they for upcoming serialization deadlines? TraceLink and independent firm Actionable Research surveyed 331 pharmaceutical manufacturers, wholesale distributors, hospitals and pharmacies to understand the challenges of lot-level compliance, and what they mean for the industry’s ability to move to a serialized world.
Watch this on-demand webinar to learn:
- How complex customer and supplier relationships have impacted compliance.
- Why many companies rely on paper rather than electronic exchange of T3 documentation.
- How trade partner custom requests have driven system modifications.
- How the experiences of pharma companies, distributors, and dispensers have varied.
- What percentage of companies have been unable to achieve compliance at all.
- What aspects of serialization pose the most risk to the industry’s success.
Presenters:
- Shabbir Dahod, President and CEO, TraceLink
- Eileen Lee, Research Consultant, Actionable Research