Table of contents
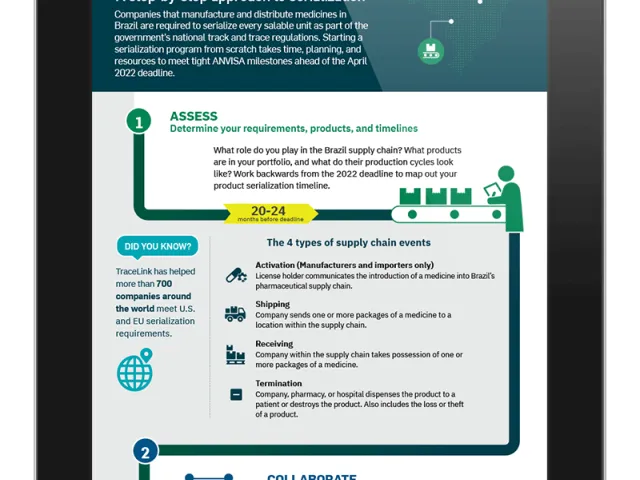
On August 20, Brazil’s Health Regulatory Agency, ANVISA, voted to approve the July 13 draft of its Normative Instruction for meeting the country’s drug serialization, traceability, and reporting requirements. The approval of the Normative Instruction is a clear statement to the industry that ANVISA expects all segments—manufacturers, distributors, and dispensers—to comply with the April 28, 2022 deadline. In response to industry questions and concerns about the deadline, ANVISA has stated repeatedly that they do not have the authority to change the date as it is written into the law and is, therefore, required to enforce the law.
The approved Normative Instruction includes the following key requirements:
- Article 4 states that by April 28, 2022, all non-exempt drugs must be serialized in order to report supply chain movements to the SNCM (National Medicine Control System).
- Article 5 states that manufacturers and importers must maintain a Serialization Plan in the SNCM portal for in-scope product lines and medicines and provide “partial percentages” of serialized production lines until all in-scope lines are ready for the mandated April 28, 2022 deadline.
- Article 6 states that by April 28, 2022, all supply chain members must report events on serialized and commercialized drugs to the SNCM.
With only 8 months before the deadline—including significant holidays and country-wide celebrations—manufacturers must act immediately to avoid possible fines, audits, or other penalties after April, 2022.
Take action: What TraceLink customers can do now
What can TraceLink customers do now to prepare for Brazil’s traceability requirements?
- Register their medicines with ANVISA and begin uploading their product and company master data required for SNCM reporting. Registry holders must enter and maintain product master data, commercialization information, and additional metadata such as Anatomical Therapeutic Chemical (ATC) codes.
- Begin integrating workstreams between internal packaging lines, contract manufacturers (CMOs), internal warehouses, and third-party logistics providers (3PL). Integration projects can—and should—be started immediately to develop and implement the appropriate transaction software and ensure secure partner on-boarding and connectivity.
- Work with TraceLink solution consultants to map use cases and identify workflow triggers for Activation and Shipment reports.
- Work with TraceLink’s Network Success Team to onboard and connect their trading partners. Companies that are downstream from manufacturers and importers will need to acquire the data from their upstream partners in order to fulfill their own reporting requirements.
- Join TraceLink’s bi-weekly Brazil Special Interest Group and upcoming Brazil Innovation Forum to discuss use cases, workflows, and ideas for product feature enhancements.
The race is on in Brazil. Don’t risk falling behind.
With so much at stake and time running out, many TraceLink customers did not wait for final approval of the Normative Instruction to get started on designing and developing their Brazil compliance solution. For companies that have not yet begun their projects, the compressed timeline means that any further delay may increase the risk of falling further behind and missing the deadline.
TraceLink offers the proven compliance solutions and local support manufacturers need to be sure that they—and their affiliates—can meet Brazil’s complex serialization, traceability, and reporting requirements by April, 2022. And only TraceLink gives manufacturers the ability to Integrate Once, Interoperate with Everyone™ to quickly connect their CMOs and 3PLs and build a downstream partner network without costly, time-consuming integrations.
Contact TraceLink to schedule a Brazil Readiness Workshop today.