761 total articles
Filter

Case Study
Case Study: CPL | The CMO Serialization Perspective—Utilizing a Standardized Approach for Efficient Partner Onboarding
View More


Case Study
Case Study: TraceLink | Building the TraceLink Network One Business Process Ecosystem at a Time
View More

Case Study
Case Study: TraceLink | FDA Pilot - Product Traceability Under 2023 DSCSA Regulations - A Business Process-Led Design for a Blockchain Network
View More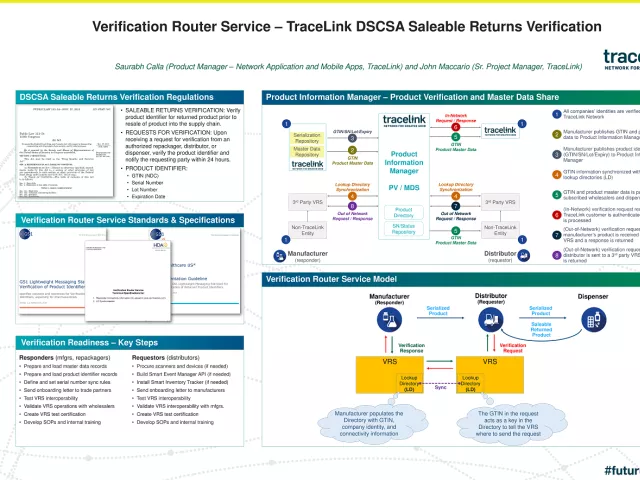
Case Study
Case Study: TraceLink | Verification Router Service - TraceLink DSCSA Saleable Returns Verification
View More
Case Study
Case Study: Almirall | Organizational Changes to Cope with Worldwide Falsified Medicines Regulations
View More
Case Study
Case Study: TraceLink | Partner with TraceLink to Achieve Compliance and Highlight Your Success
View More
Case Study
Case Study: Value Drug Company | DSCSA Product Investigation—A Compliance Solution
View More
Video
Case Study: PharmaLink | Closing the Gap on Cradle-to-Grave Traceability via Reverse Distribution and EPCIS
View More
eBook
Why An Open, Standards-Based Approach is Essential for the Pharmaceutical Supply Chain
View More

Featured Webinars
TraceLink Q&A: What Does Enforcement Discretion Mean for Your Saleable Returns Strategy?
View More
Blog
From Crypto Codes to Complex Reports: Expert Advice on TraceLink’s Russia Compliance Solution
View More

Case Study
Orient Pharma and TraceLink: Partnering for Success on Serialization and Quality
View More














