Case Study
Thank you for contacting us; we’ll be in touch shortly.

Pharma Drops Vendor over Integration and Validation Failures, Joins TraceLink
One brand owner is now able to make up for lost time, maintain security, and move ahead in their mission to deliver quality and safety. Find out what…

Case Study: Ferrer | Building a Master Data Strategy for EU FMD
Learn how Ferrer worked with TraceLink to manage its master data for EU FMD compliance.
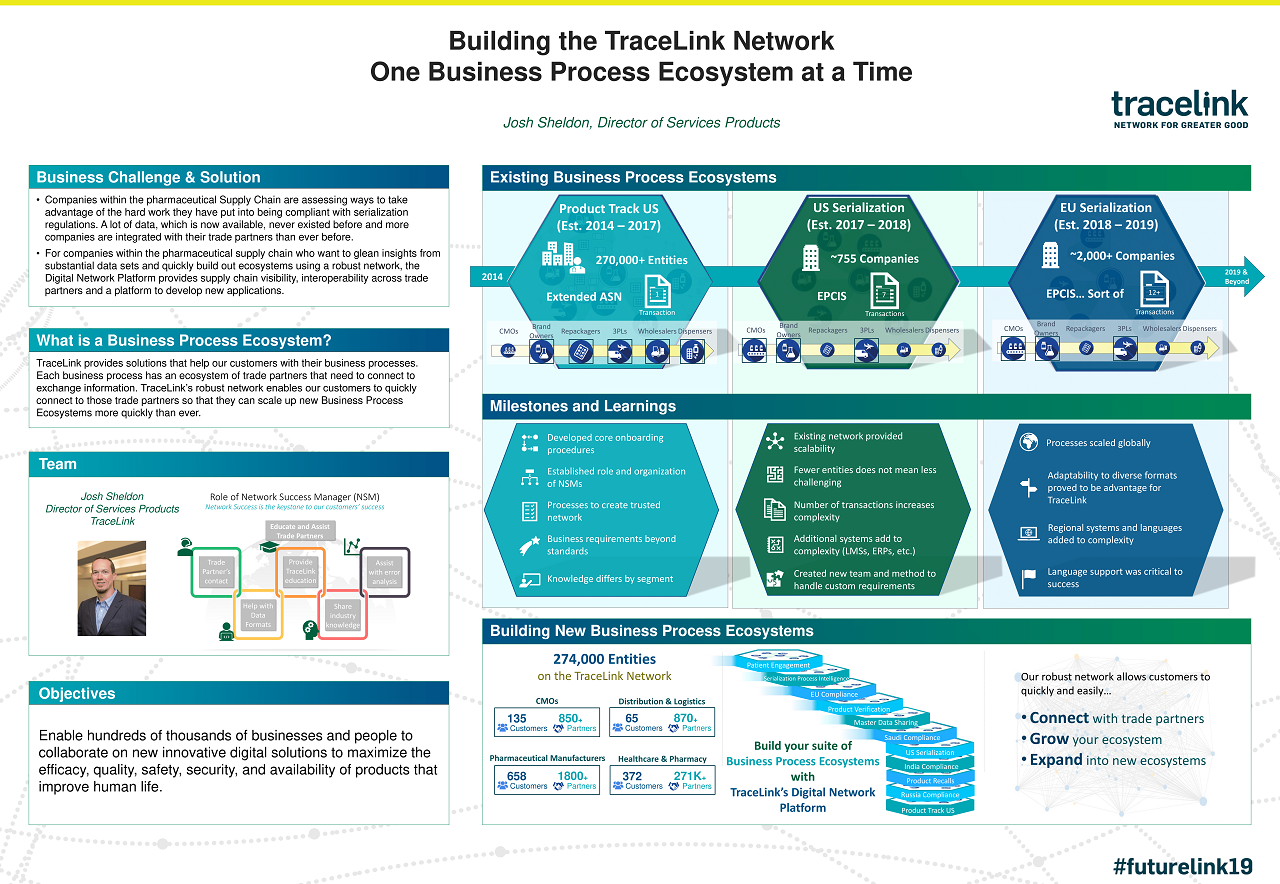
Case Study: TraceLink | Building the TraceLink Network One Business Process Ecosystem at a Time
See how TraceLink's powerful digital supply network enables customers to quickly connect to trade partners for scaling up business process ecosystems.
Spanish Pharma Discovers All Its Partners Are Already On TraceLink Network
Read how a small pharma company discovered it could connect to all of its trade partners with one connection to TraceLink.

Case Study: IBSA | Using Serialization to Ensure Product Integrity
Learn how IBSA used serialization to protect their product from counterfeiting.
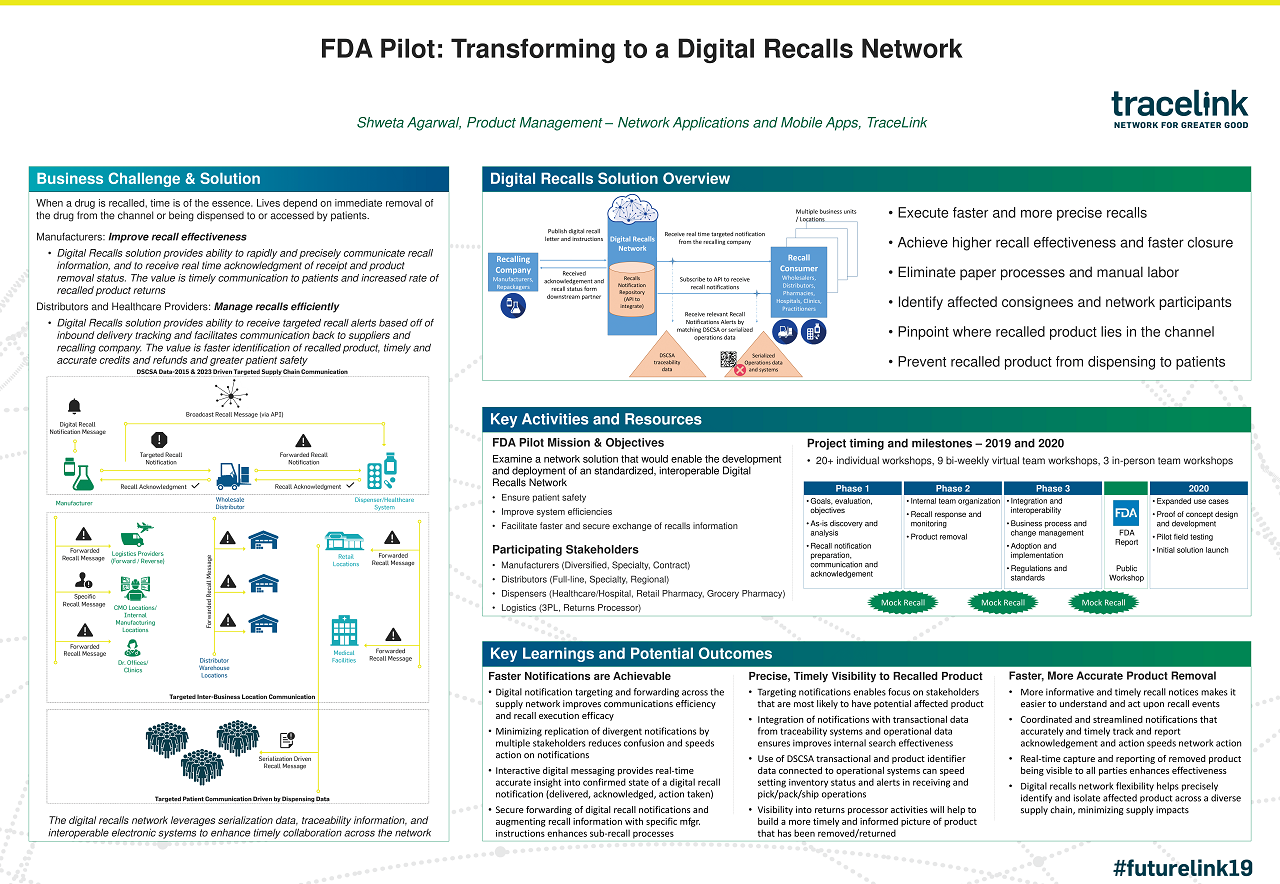
Case Study: TraceLink | FDA Pilot - Transforming to a Digital Recalls Network
Find out how TraceLink helps pharmaceutical manufacturers and dispensers manage recalls more quickly and efficiently than ever.

IBERFAR: Partnering with TraceLink for FMD and Digital Integration
Hear the IBERFAR team explain how they mastered serialization and FMD with TraceLink.

Case Study: Medreich | EU FMD from Project Plan to Post Implementation
See how Medreich and TraceLink collaborated to achieve EU FMD compliance.

Case Study: TraceLink | FDA Pilot - Product Traceability Under 2023 DSCSA Regulations - A Business Process-Led Design for a Blockchain Network
TraceLink's breakthrough blockchain solution, Trace Histories, can help pharma customers comply with US DSCSA regulations that go into effect in 2023.

Virtual Manufacturer Streamlines Software Validation with AVM
Read why a leading oncology-focused biotechnology company chose TraceLink's Automated Validation Manager (AVM) to reduce software validation…

Case Study: Medreich | Anti-Tampering and EU FMD
Learn how Medreich designed an EU FMD-compliant label to work with anti-tampering devices.
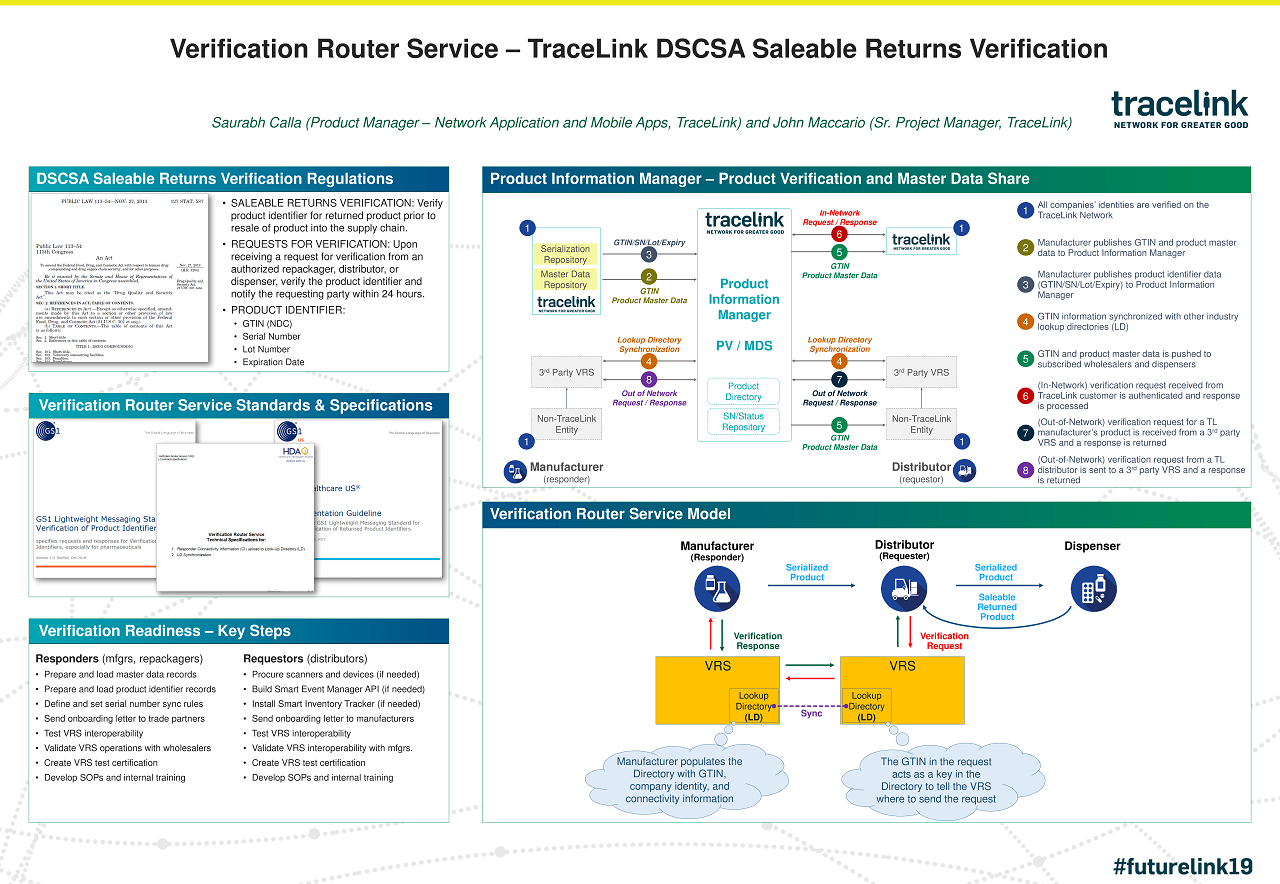
Case Study: TraceLink | Verification Router Service - TraceLink DSCSA Saleable Returns Verification
TraceLink helps customers meet DSCSA saleable returns verification requirements via the Verification Router Service model. See how.


