Global Track & Trace
Thank you for contacting us; we’ll be in touch shortly.
An Overview of EU FMD
Understand the basics of EU FMD and what you need to do to comply by watching this 12-minute on-demand webinar with one of our experts.
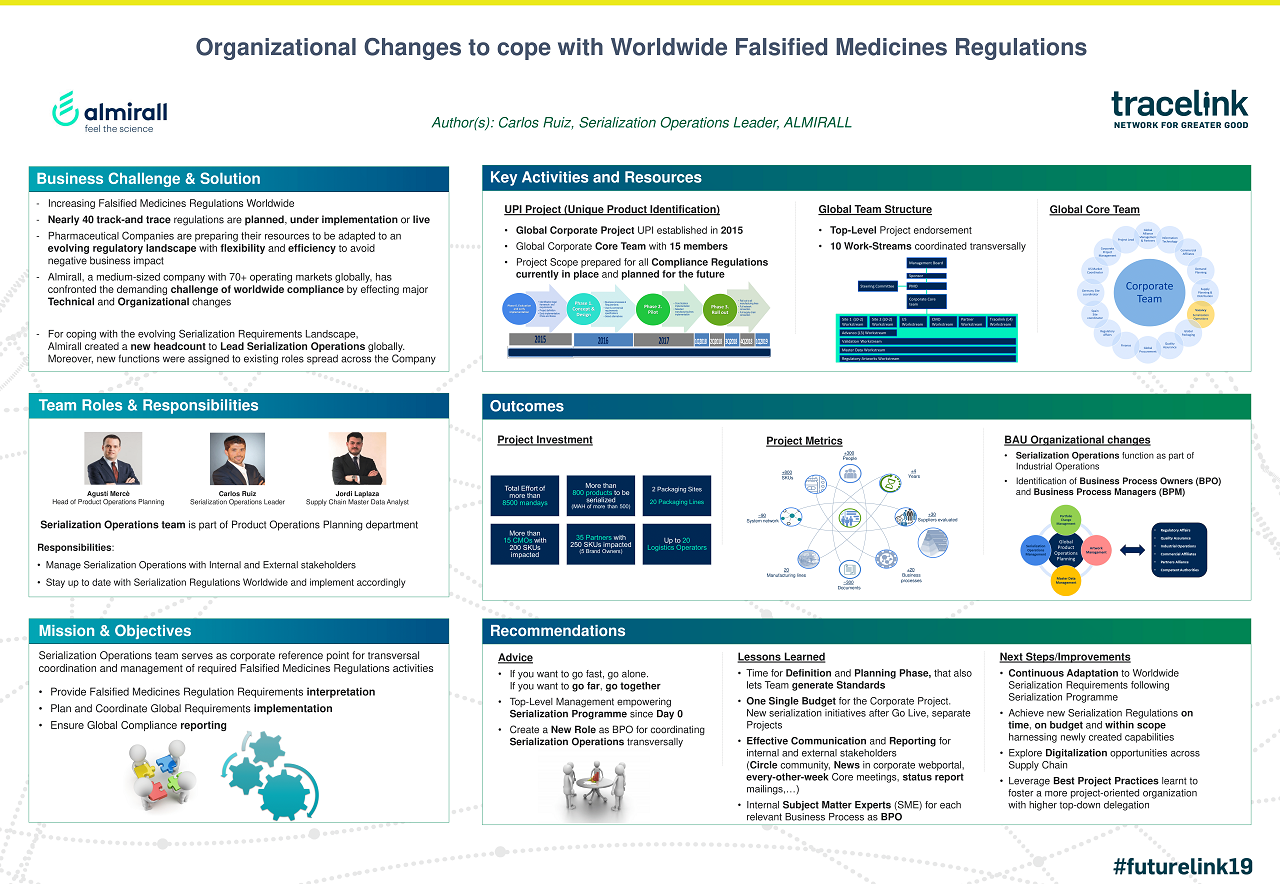
Case Study: Almirall | Organizational Changes to Cope with Worldwide Falsified Medicines Regulations
See how TraceLink helped customers like Almirall implement global compliance strategies to comply with worldwide falsified medicine regulations.

AVM Helps Virtual Manufacturer Automate Software Validation Across Diverse Supply Chain
Read how a leading biopharmaceutical manufacturer turned to TraceLink’s Automated Validation Manager to help manage software compliance.

Virtual Manufacturer Streamlines Software Validation with AVM
Read why a leading oncology-focused biotechnology company chose TraceLink's Automated Validation Manager (AVM) to reduce software validation…

Validation and Automated Validation: Top Industry Questions
Get 27 answers covering the impact of validation to your requirements, resources, and risk—and how automation offloads the entire burden.

How Do I Upload Aggregated Data to the European Hub?
If your business is aggregating, you need to know how to upload your product data once EU FMD comes into effect. Find out here.

Horizontal Integration: Why It’s Essential for Brazil Compliance
Success in the Brazilian market will depend on horizontal integration: end-to-end data exchange between you and your trading partners. Learn why.

From Crypto Codes to Complex Reports: Expert Advice on TraceLink’s Russia Compliance Solution
Get expert advice on meeting Russia's complex track and trace requirements for pharmaceutical companies. Learn how TraceLink can help.

Getting Started: EU FMD Guide to Pharma Serialization
Get started with understanding EU FMD regulations and the serialization challenges ahead, with this introductory infographic guide.

Understanding the Serialization Challenge: The Actelion Story
Hear Actelion discuss the importance of track and trace, how they approached the serialization challenge, and why they believe you can’t start too…

Russia Regulatory Updates
View a compilation of the most recent track and trace regulations for the healthcare supply chain in Russia. Get insights into compliance updates.

Will My Current China Reports Work with the New Traceability Systems?
Learn how China’s amended Drug Administration Law expands the scope of NMPA reporting requirements and impacts your company’s serialization strategy.


