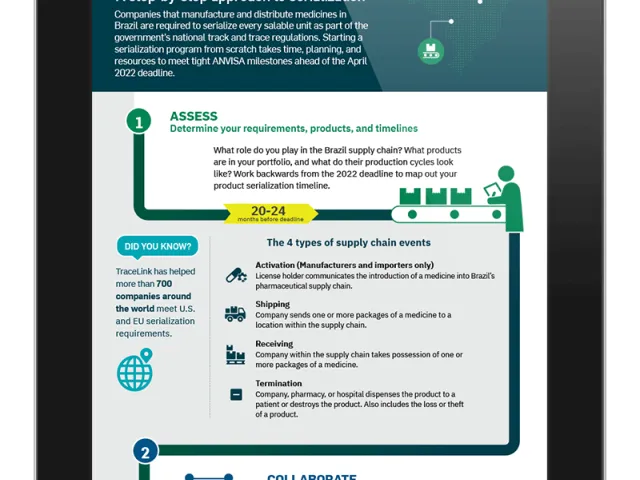
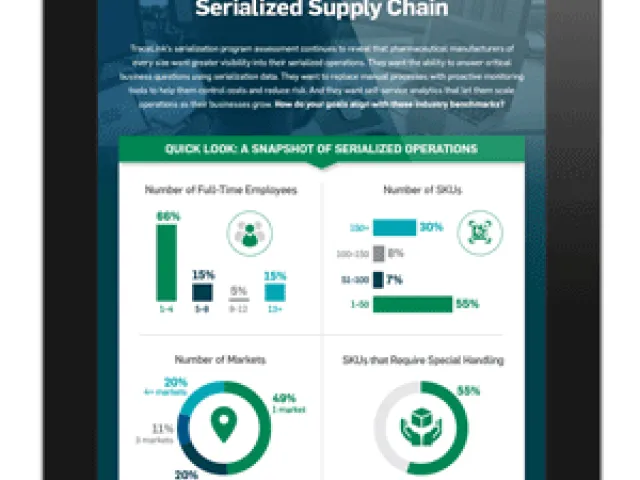
Table of contents
Key Takeaways from the August 2021 Brazil Special Interest Group sessions
- On August 20, ANVISA approved the Normative Instruction, which confirms the April 28, 2022 deadline for manufacturers, wholesalers, and dispensers to serialize products and report events for those products entering commercialization on or after the deadline.
- Approval of the Normative Instruction is expected to facilitate discussions with ANVISA on clarifying questions on multiple reporting use cases, including imported products.
- Tracelink has developed a survey for its customers to use to assess the readiness of third-party logistics providers (3PLs) that they plan to authorize as proxies for reporting to SNCM, ANVISA’s traceability system.
Approval of Normative Instruction confirms April deadline
With its approval of the Normative Instruction on August 20, Brazil’s Health Regulatory Agency, ANVISA, is sending a clear message that it expects all segments—manufacturers, importers, distributors, and dispensers—to comply with the April 28, 2022 deadline. ANVISA has reiterated that the agency does not have the authority to change the date as it is written into the law.
TraceLink’s Brazil Director, Luca Gabrielli, noted that ANVISA has acknowledged the complexity of implementing the serialization and track and trace capabilities but has reiterated that, as a regulatory body, it’s bound by the existing law that defines April 28, 2022 as the date by which products must be serialized and reporting to SNCM must begin.
The impact on manufacturers and importers
Luca Gabrielli summarized the key provisions from the approved Normative Instruction:
- All supply members must meet the requirements of the SNCM, including wholesalers and dispensers. As a result, manufacturers’ downstream partners will require serialization and aggregation information for received shipments in order to fulfill their reporting obligations.
- The ANVISA registry holder (manufacturer or importer) is required to provide product master data to the SNCM for current products licensed in Brazil. Master data for new products must be provided as they are launched.
- The date by which products must be serialized before they can enter the Brazil market is April 28, 2022.
- The date after which events must be reported to the SNCM is April 28, 2022.
- Manufacturers must submit a Serialization Plan within 30 days of the SNCM system becoming available that outlines how they will implement serialization and reporting for Brazil products.
- Manufacturers and Importers are expected to adapt their Brazil compliance solution as ANVISA continues to develop the SNCM system and reporting processes.
In response to member questions around reporting requirements, Luca Gabrielli pointed out that the reporting requirement applies only to products serialized after April 28, 2022 and that, with the approval of the Normative Instruction, ANVISA is expected to provide more clarification around requirements for a number of unaddressed business use cases, such as reporting on imported products.
Finally, Luca Gabrielli noted that ANVISA can impose penalties on companies that are not in compliance, including fines; shutting down a production or distribution facility; and initiating an audit of the manufacturer.
Assessing 3PL readiness: Questions manufacturers and importers should ask
In his presentation, TraceLink project manager Greg Firestone noted that some companies may choose to designate a third-party logistics provider (3PL) as a proxy for reporting to SNCM. TraceLink is providing an assessment to its customers to ensure that a 3PL has the capabilities and infrastructure to keep the delegating company compliant. The survey covers 5 key areas:
- Data Management
- Validation
- Proxy Reporting Capabilities
- Horizontal Data Exchange
- Disaster Recovery/Business Continuity
Greg Firestone also pointed out that TraceLink compliance customers can—and should—be configuring their core Serialized Operations Manager application with Brazil product and partner master data to be ready when the Brazil Compliance module is released in September.
Stay informed with TraceLink’s Brazil Special Interest Group and Product Innovation Forum
TraceLink's Brazil Special Interest Group will soon convert to a Product Innovation Forum where TraceLink customers can discuss business requirements and get updates on TraceLink’s Brazil Compliance and Serialized Traceability solutions for horizontal data exchange. TraceLink’s Brazil Community meets every two weeks on Thursdays. Join the TraceLink Community.