Regulatory/Compliance
Thank you for contacting us; we’ll be in touch shortly.
DSCSA Risks & Regulations for Hospitals and Pharmacies
View our infographic to find out how to comply with federal regulations for drug purity to stay DSCSA compliant now and down the road.

How Do I Prepare Product Master Data for EU FMD?
Learn what product master data you need to upload to the European Hub under EU FMD, and how to go about preparing it.

AVM Helps Virtual Manufacturer Automate Software Validation Across Diverse Supply Chain
Read how a leading biopharmaceutical manufacturer turned to TraceLink’s Automated Validation Manager to help manage software compliance.

Digital Transformation of the Supply Chain eBook
Industry snapshot: Find out how digital transformation will lead to fewer drug shortages and better patient outcomes.

Saleable Returns: DSCSA and Trade Partner Requirements Explained
View this on-demand webinar on saleable returns, DSCSA requirements and the role of master data.
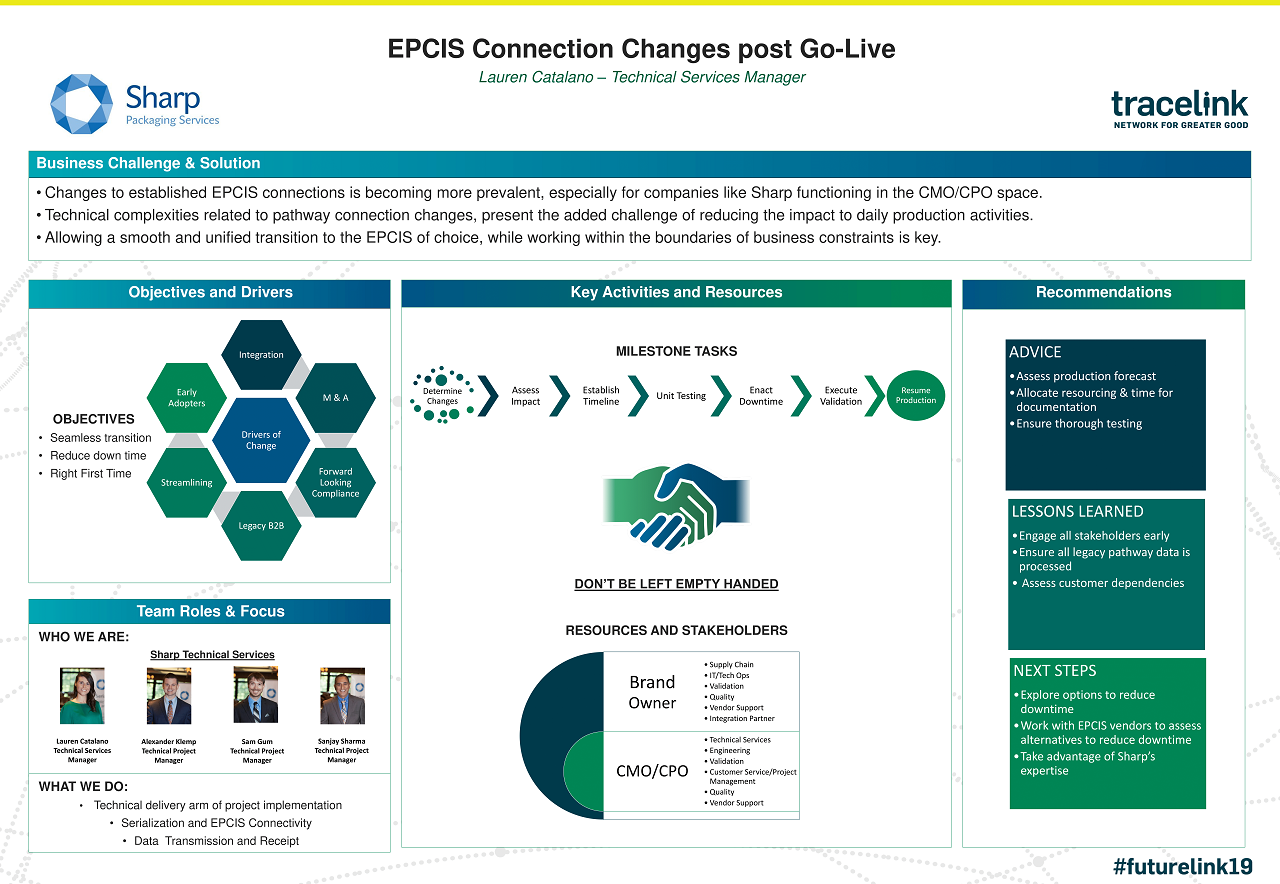
Case Study: Sharp Packaging Services | EPCIS Connection Changes Post Go-Live
See how Sharp Packaging Services overcame EPCIS change management challenges in the pharma supply chain with TraceLink's help.

China Regulatory Updates
View a compilation of the most recent track and trace regulations for the healthcare supply chain in China. Get insights into compliance updates.

Horizontal Integration: Why It’s Essential for Brazil Compliance
Success in the Brazilian market will depend on horizontal integration: end-to-end data exchange between you and your trading partners. Learn why.

French Pharma Selects TraceLink for Smooth LMS Integration, Lower Costs
Learn what issues one pharma company faced with other solution providers, and why it ultimately chose TraceLink for EU FMD compliance.

80 Must-Know Serialization Terms for U.S. Wholesale Distributors
Learn the language of wholesale distributor serialization to accelerate your compliance journey.

FDA Announces Enforcement Delay for Manufacturers But Law Still in Effect
Understand what the announcement means for pharma companies as the November 2017 deadline approaches.
The Path to Building Supply Chain Agility in Healthcare
Hear from global supply chain thought leaders as they discuss the journey to an agile, resilient, and reliable supply chain.


