Regulatory/Compliance
Thank you for contacting us; we’ll be in touch shortly.

Preparing for Saudi Arabia Compliance Reporting
TraceLink SVP of Product Marketing, Lucy Deus, provides an in-depth review of the January 2019 Saudi reporting requirements for pharma supply chains.

Burlington Drug Company Partners with TraceLink
Hear wholesale distributor Burlington discuss how DSCSA lot level requirements have changed their business and how TraceLink has helped.

Operational Efficiency Matters: Why Knox Community Hospital Changed DSCSA Solutions
Not all DSCSA compliance solutions are created equally. That’s what Knox Community Hospital discovered the hard way, after struggling with “an…

Transforming the Retail Pharmacy Supply Chain Through DSCSA On-Demand Webinar
Watch this on-demand webinar to learn how retail pharmacies are using DSCSA as a catalyst for supply chain transformation.

Pharma Drops Vendor over Integration and Validation Failures, Joins TraceLink
One brand owner is now able to make up for lost time, maintain security, and move ahead in their mission to deliver quality and safety. Find out what…

Supply Chain Risk Management in the Complex Russia Compliance Market: Why Self-Service Troubleshooting Capabilities are Critical to Your Success
Find out why self-service troubleshooting with Serialized Product intelligence is critical to supply chain risk management in Russia’s complex…

Getting Started: EU FMD Guide to Pharma Serialization
Get started with understanding EU FMD regulations and the serialization challenges ahead, with this introductory infographic guide.

Saleable Returns: VRS Capabilities and Decision Criteria for Manufacturers
Learn the criteria for choosing a VRS as the foundation of a saleable returns solution, including network governance, scalability, and…

Achieving Continuous Compliance with Automated Validation Manager
Learn how TraceLink's Automated Validation Manager (AVM) helps companies meet compliance requirements and stay focused on core business objectives.

As DSCSA Manufacturer Serialization Enforcement Begins, More Than 250 TraceLink Customers Are Live
As full FDA serialization enforcement begins on November 28, TraceLink has helped more than 250 manufacturing companies go live with serialization.

An Interview with Tjoapack: Innovation through Serialization
Learn how Tjoapack turned the challenge of updating packaging for EU FMD into an opportunity for innovation, in this on-demand webinar.
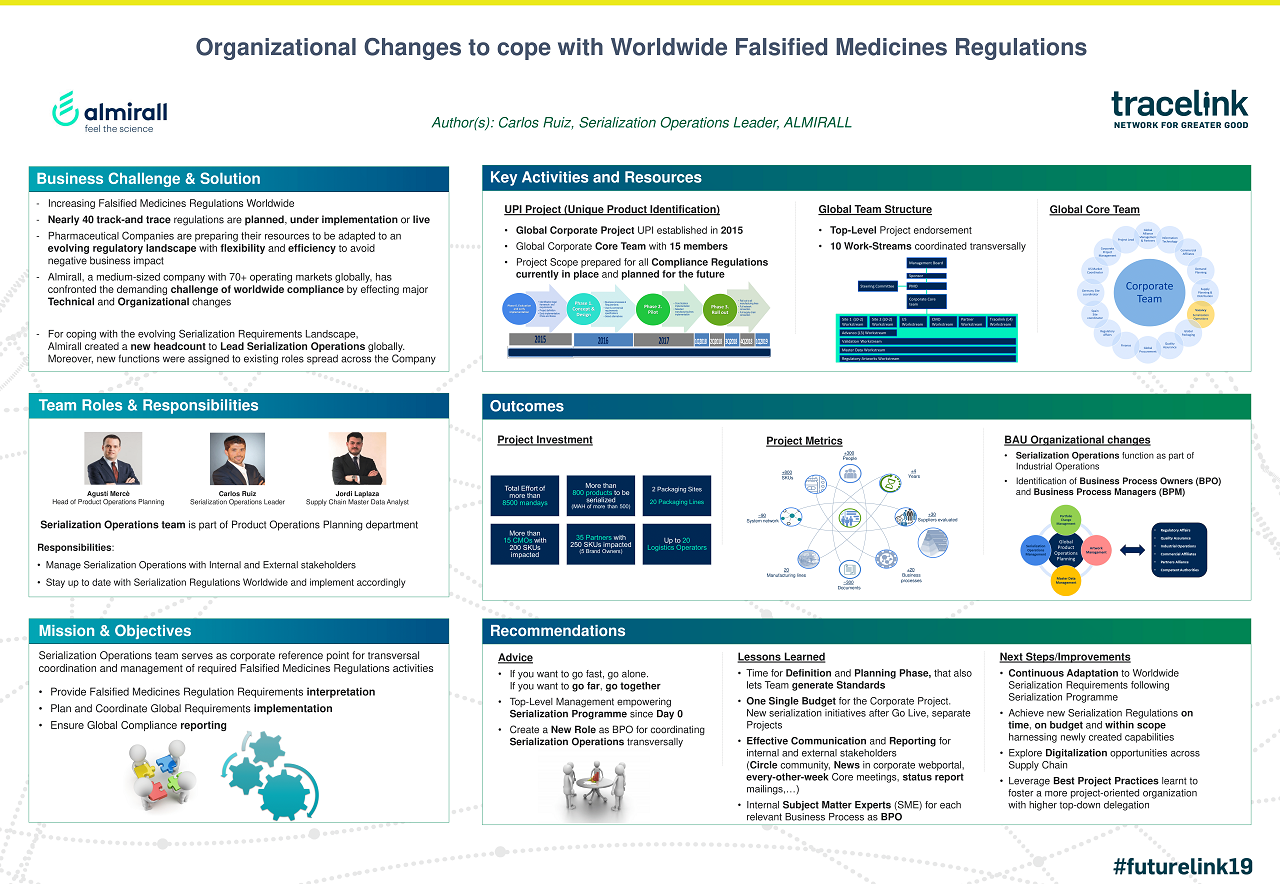
Case Study: Almirall | Organizational Changes to Cope with Worldwide Falsified Medicines Regulations
See how TraceLink helped customers like Almirall implement global compliance strategies to comply with worldwide falsified medicine regulations.


